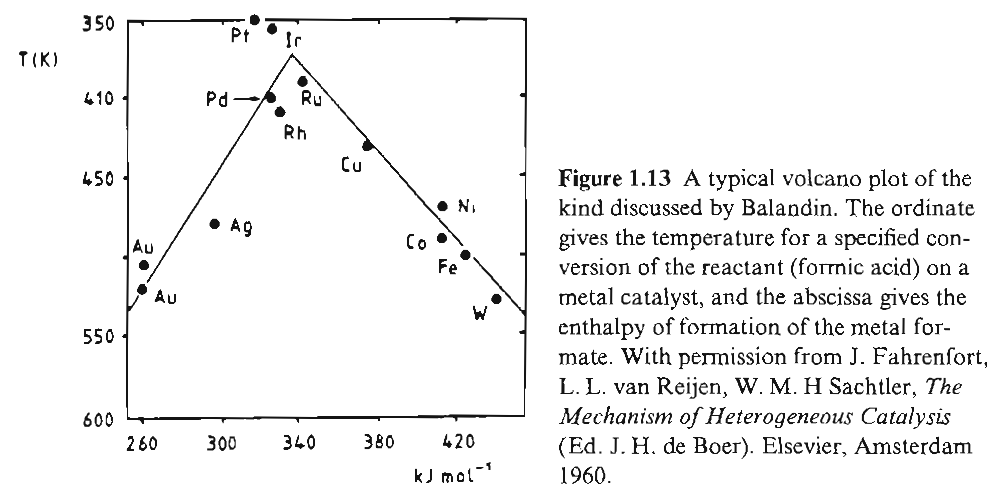
Consider reaction of formic acid decomposition:
If temperature of specified conversion over different metals is plotted against enthalpy of formation of the metal formate, a Balandin volcano plot is obtained {ISBN: 3-527-29288-8}:

Volcano shaped plots support Sabatier's idea. Too high an enthalpy of formation would not facilitate catalysis, and neither would too low a value as there would be little propensity for the intermediate compound to form.
The reaction is relatively simple, however, with only single reactant. It is also not clear whether enthalpies of formation of compounds with reaction products are important or not.
In the reaction of hydrodesulphurization of dibenzothiophene volcano plot is observed when the activity is plotted against enthalpy of the bulk metal sulphide formation {ISBN: 3-527-29288-8}:
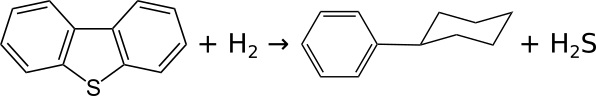
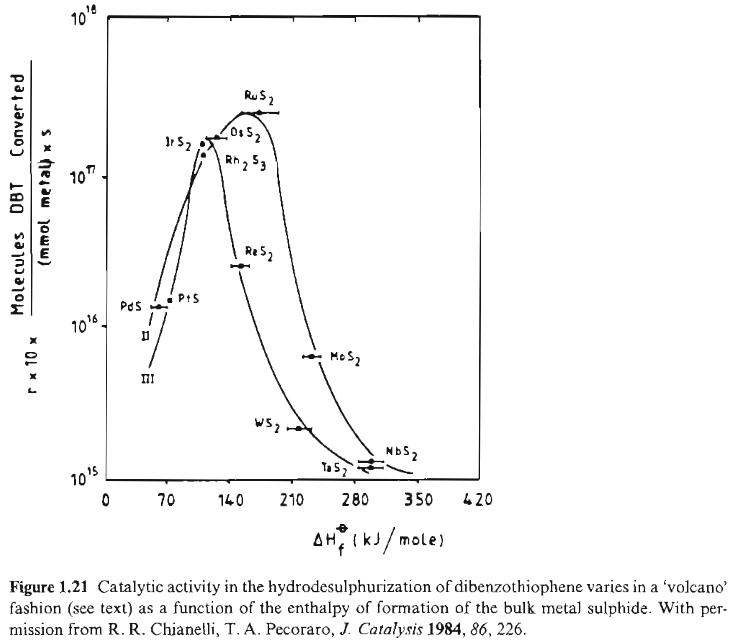
From this volcano plot we deduce that the enthalpy of formation for maximal activity takes an intermediate value: the most effective catalysts are those metal sulphides which have the ability to form and regenerate sulphur vacancies, required to create coordinative unsaturation at the metal centre.