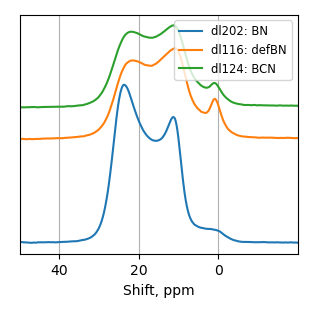
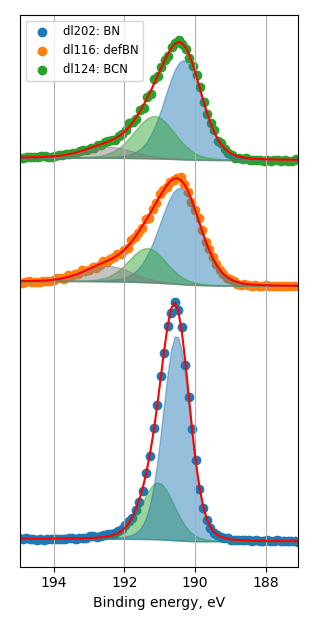
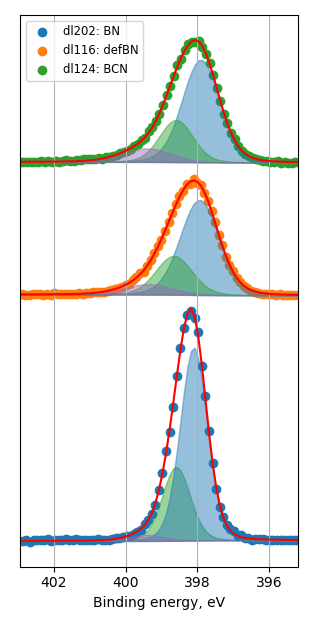
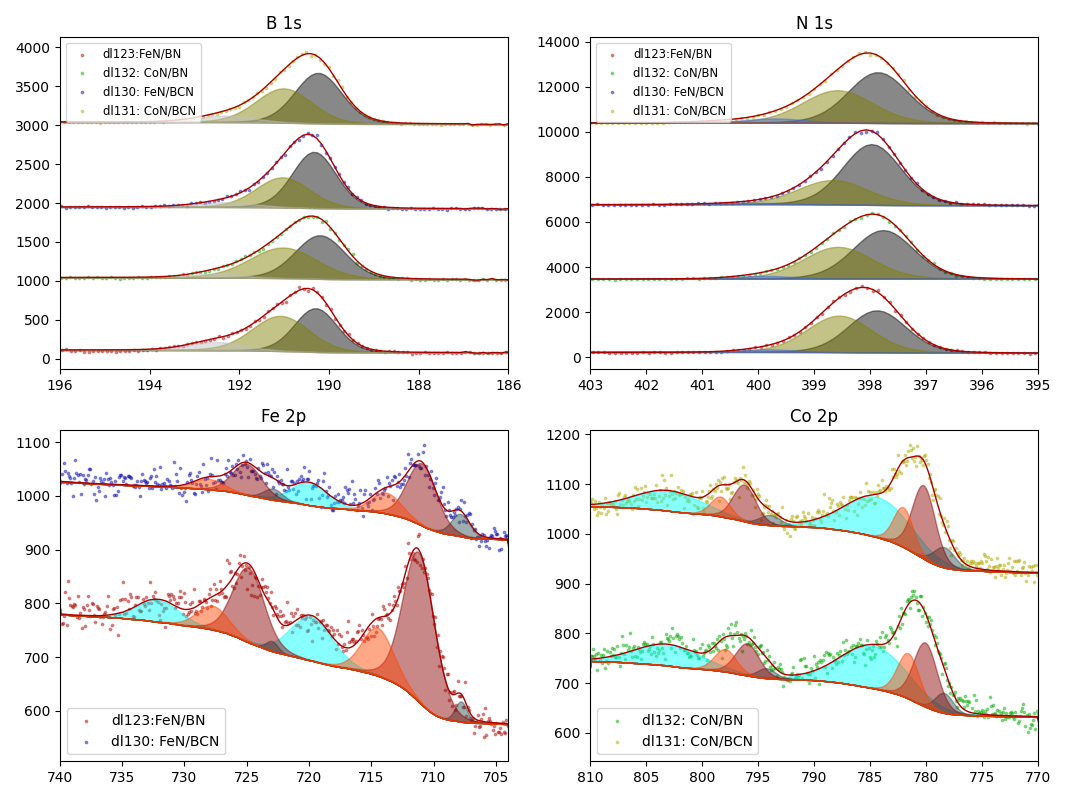
How balls/h-BN ratio of hBN ball-mill treatment influence characteristics of FeN/h-BN?
Samples were produced via Fe hydroxide chemical precipitation. Boron nitride powder was dispersed in acidified iron nitrate solution using ultrasound at 5°C. NaOH water solution was added to the suspension dropwise at 5°C at vigorous stirring using magnetic mixer. After deposition, samples were washed with water and dried in IPA at ambient conditions. Nitrides were obtained via ammonolysis in ammonia flow at 500°C.
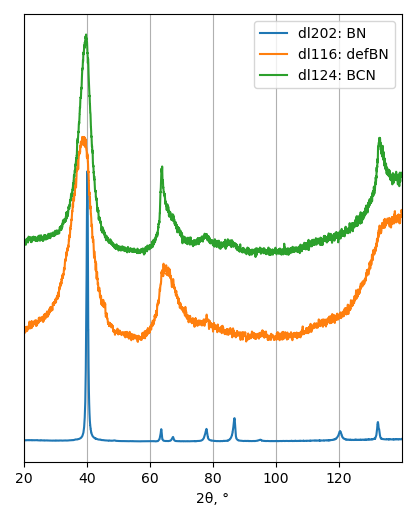
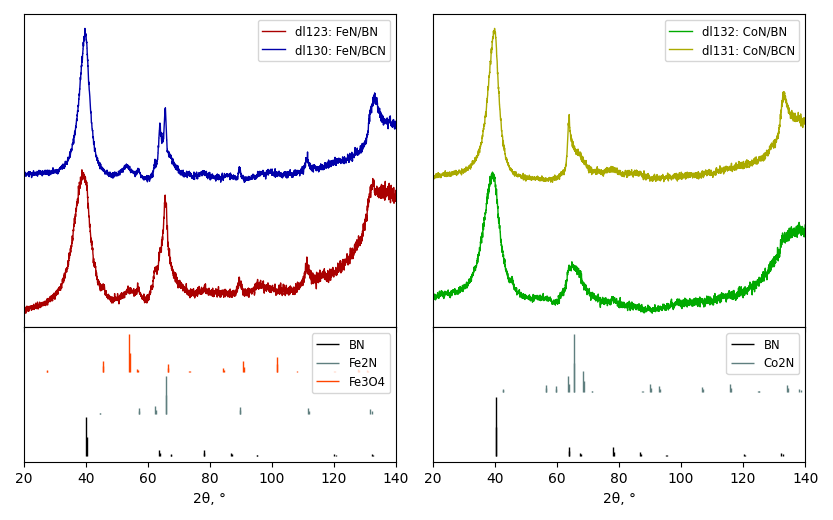
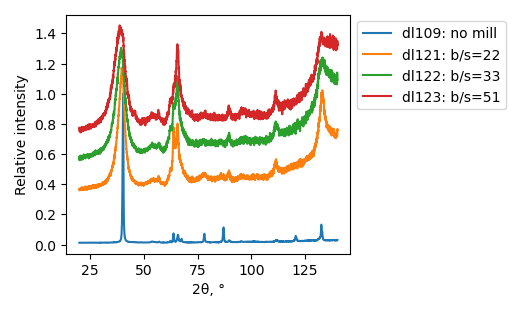
XRD
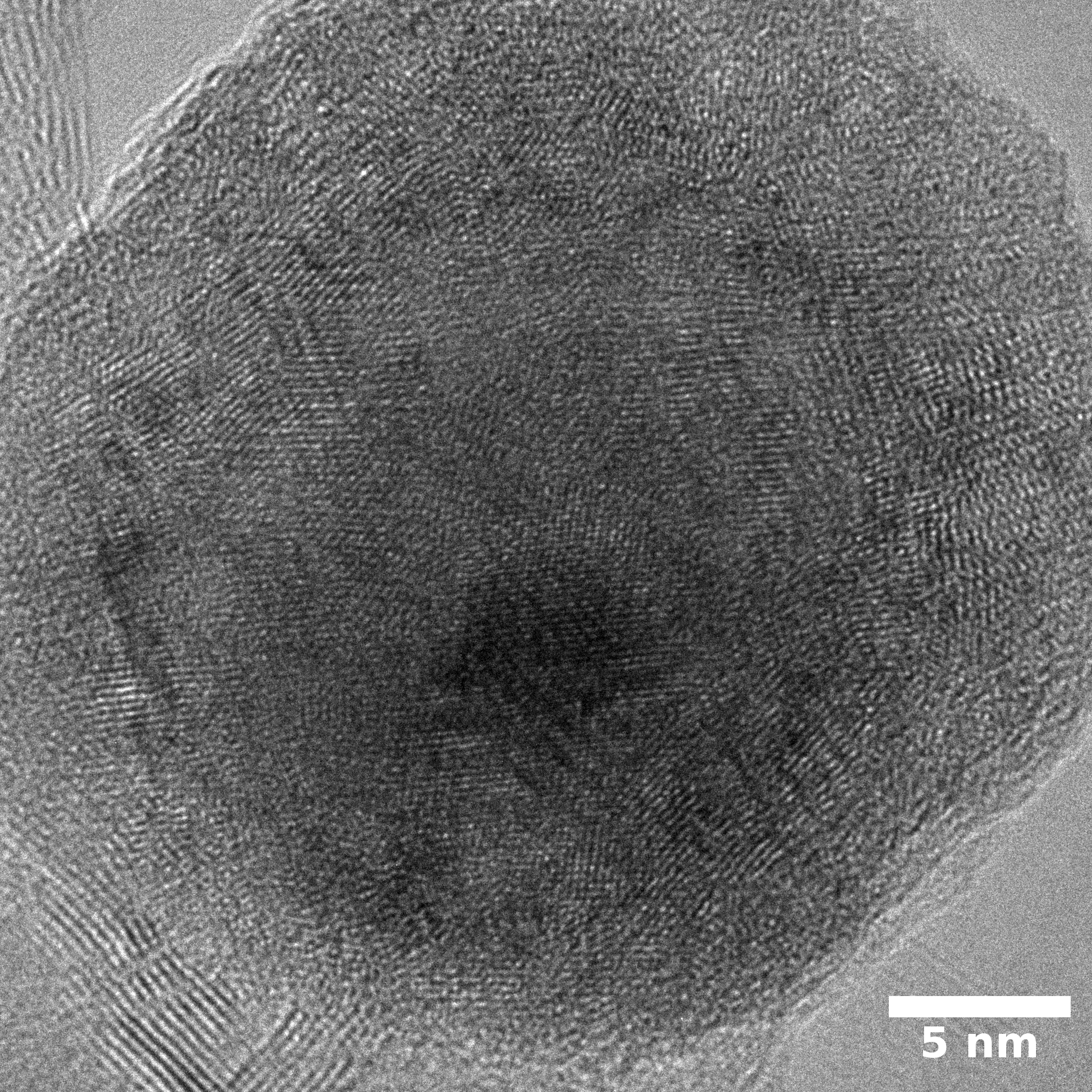
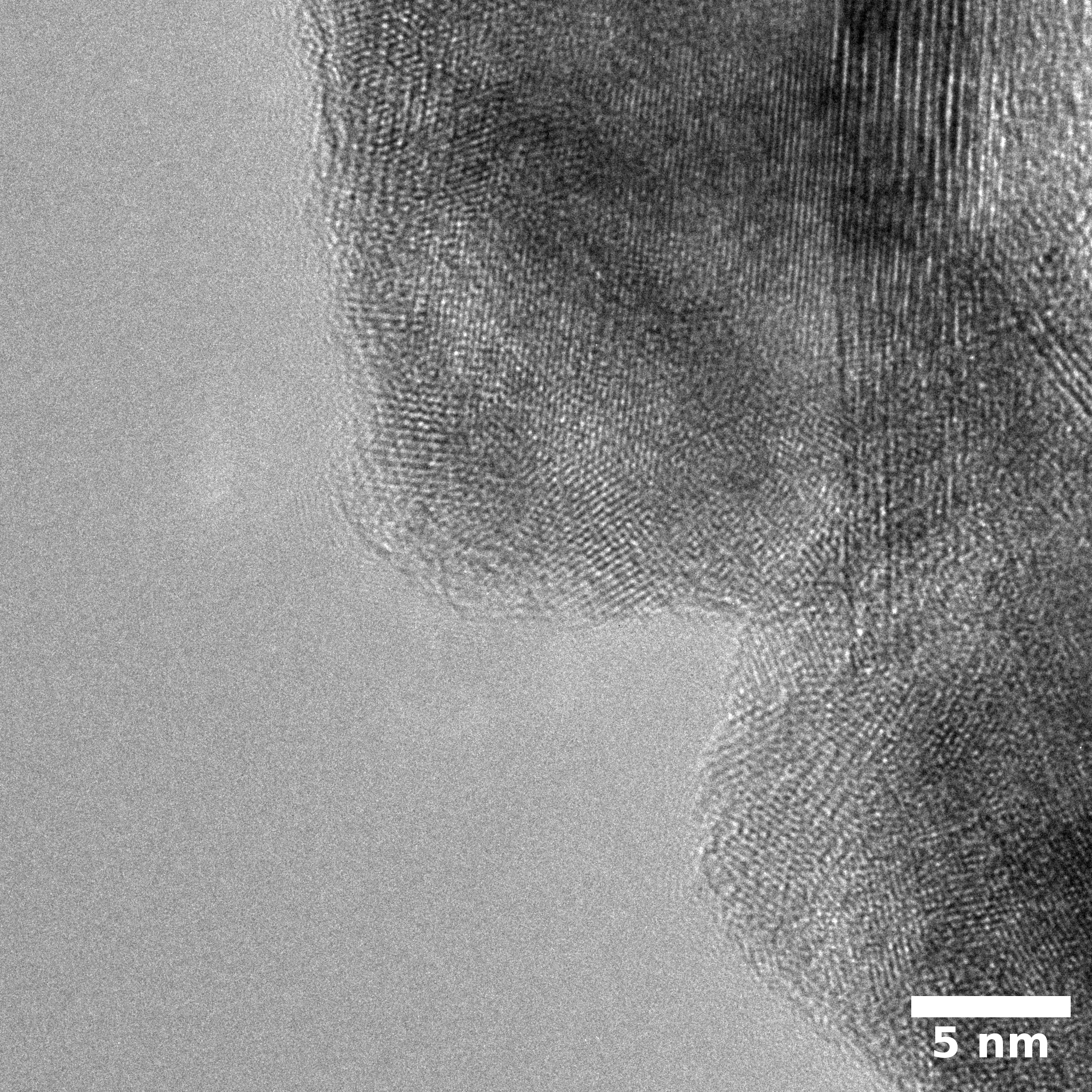
Samples consist of h-BN, Fe2N and Fe3O4 phases. There is no change in composition depending on b/s ratio. The difference is only in FWHM of h-BN peaks.
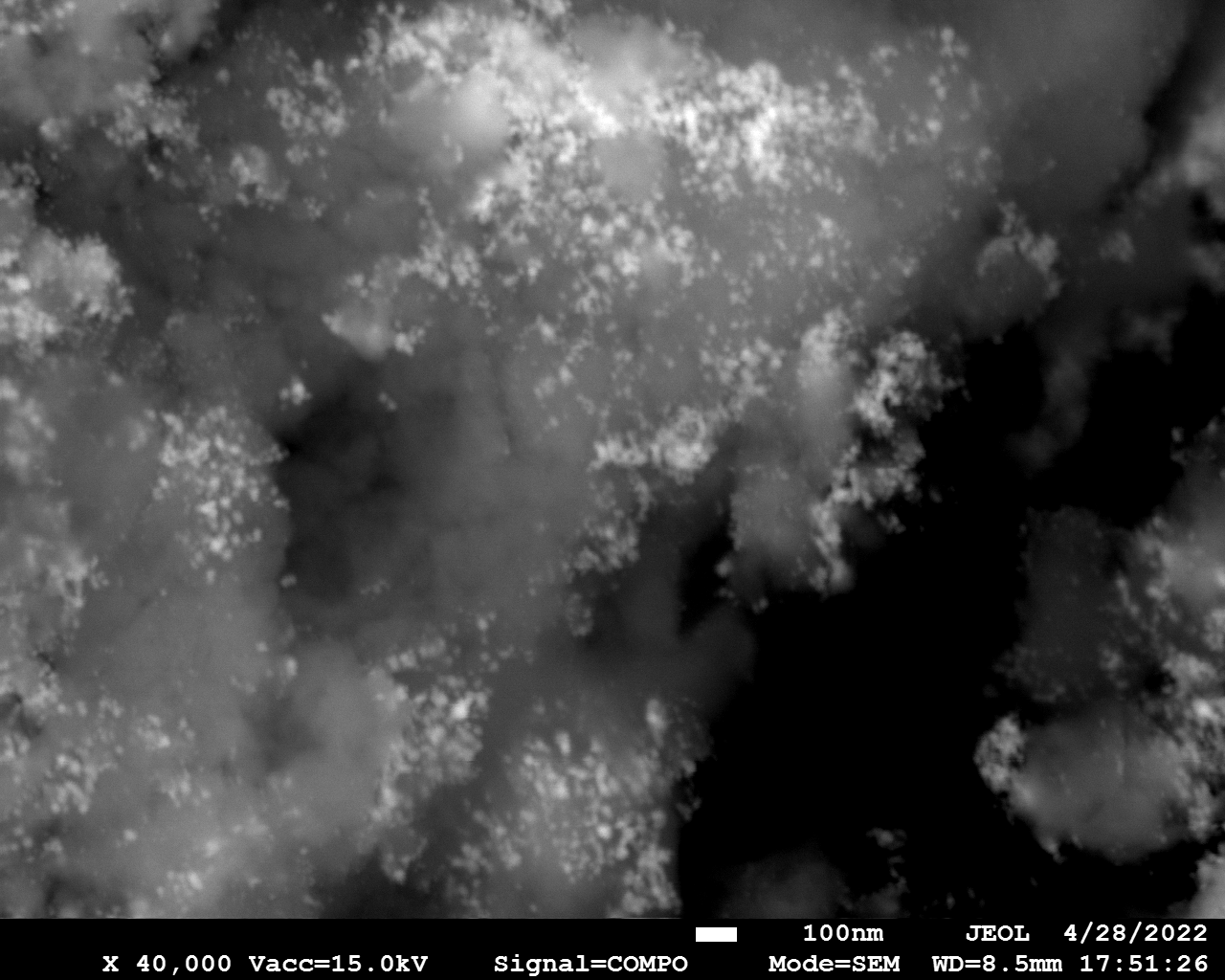
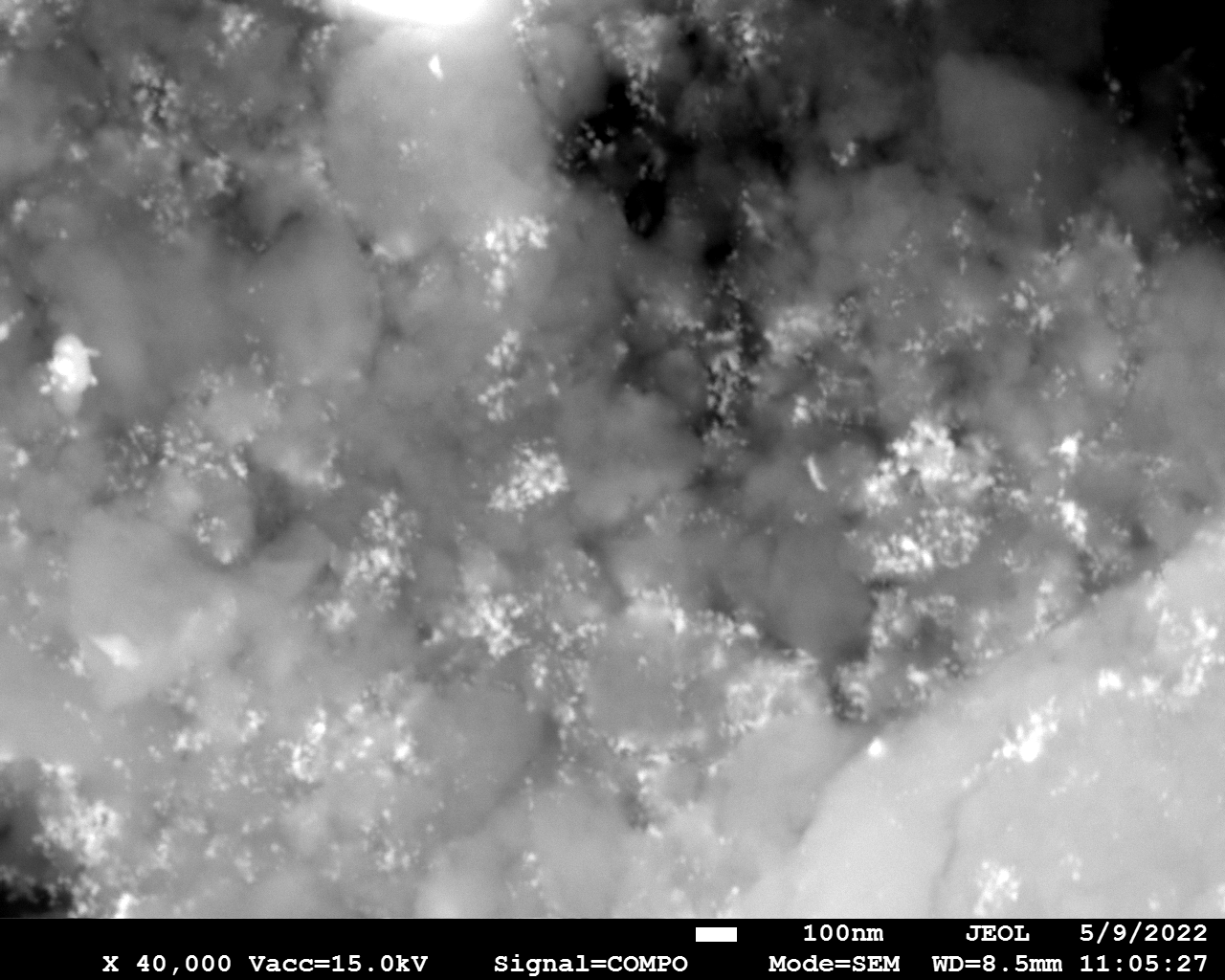
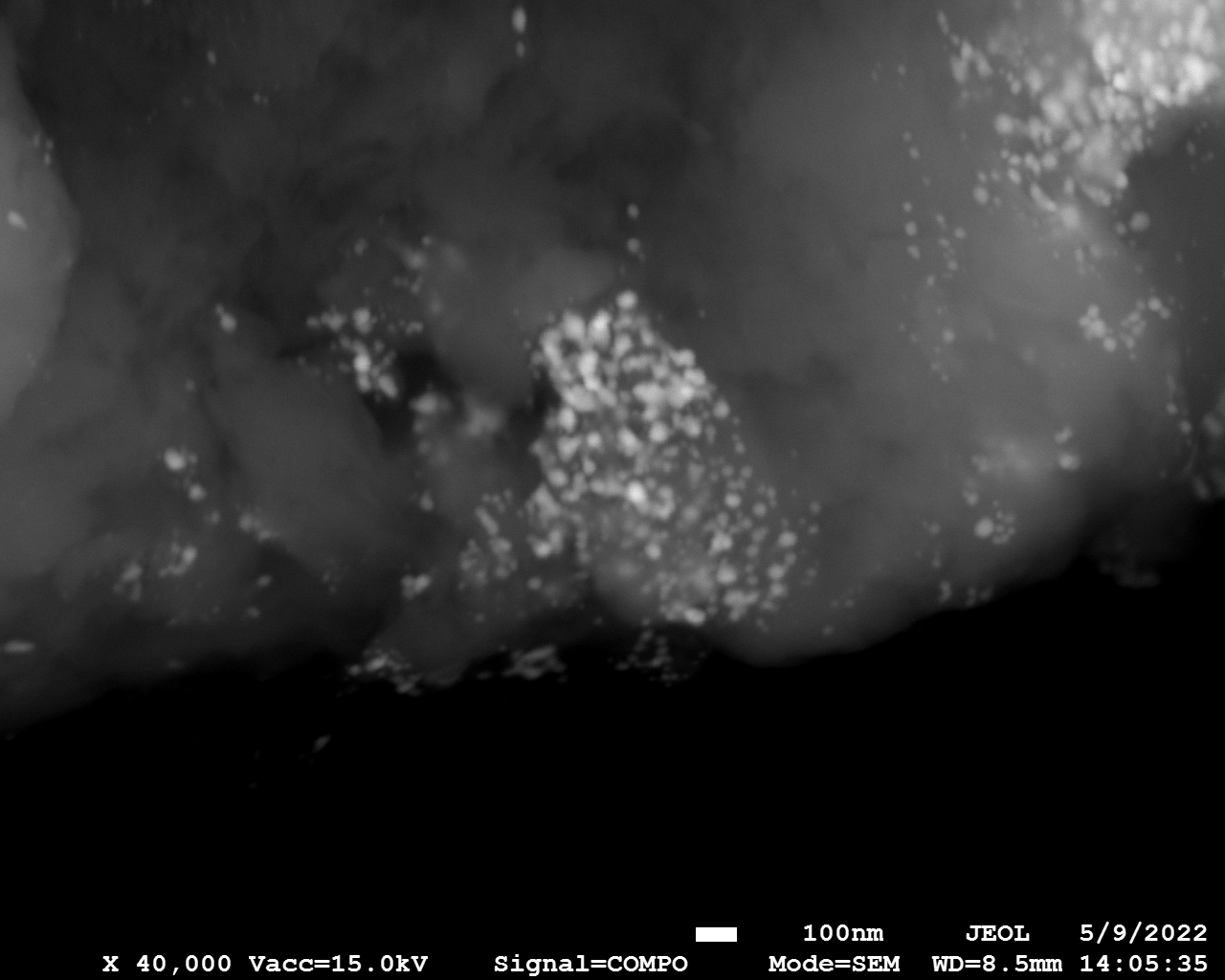
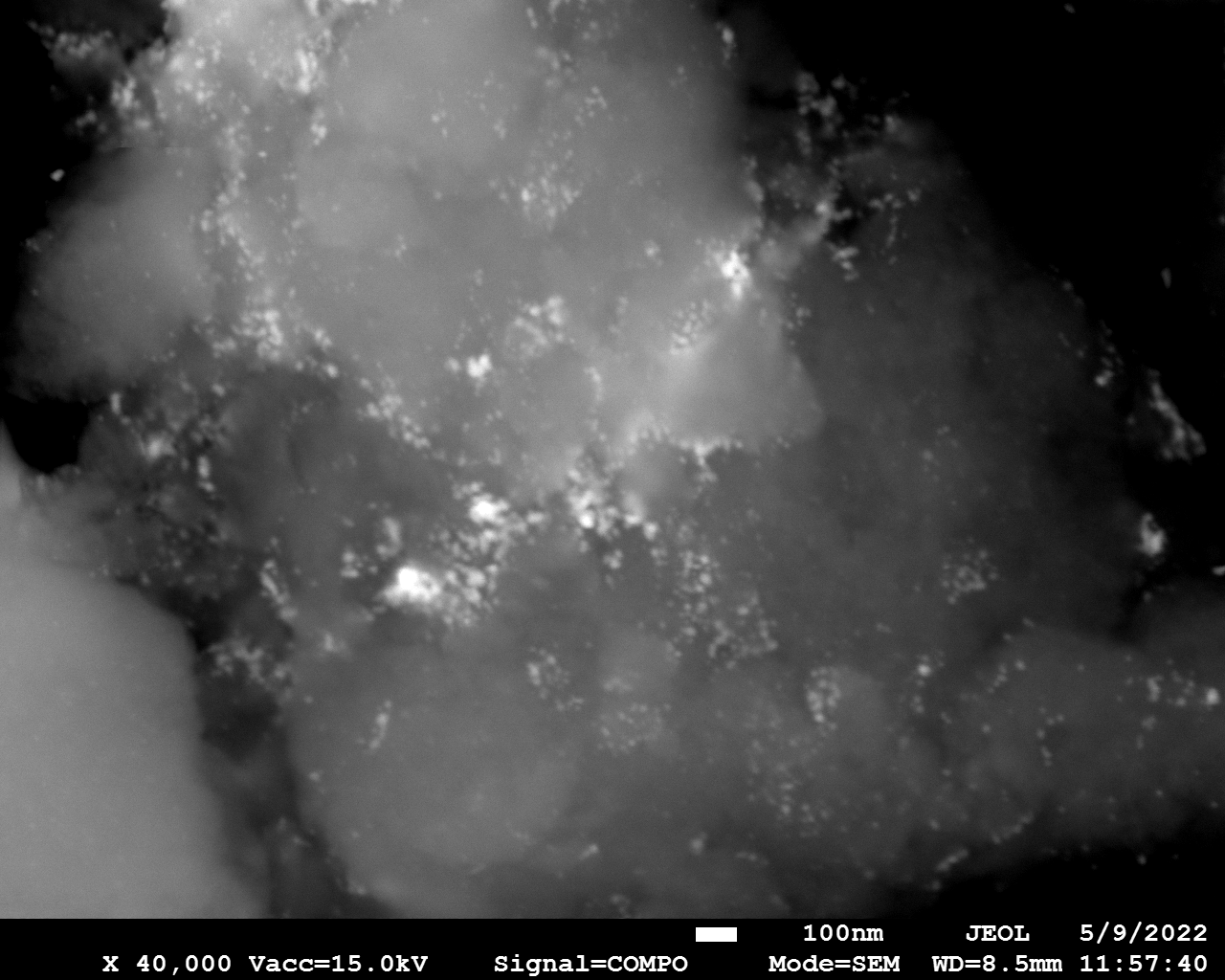
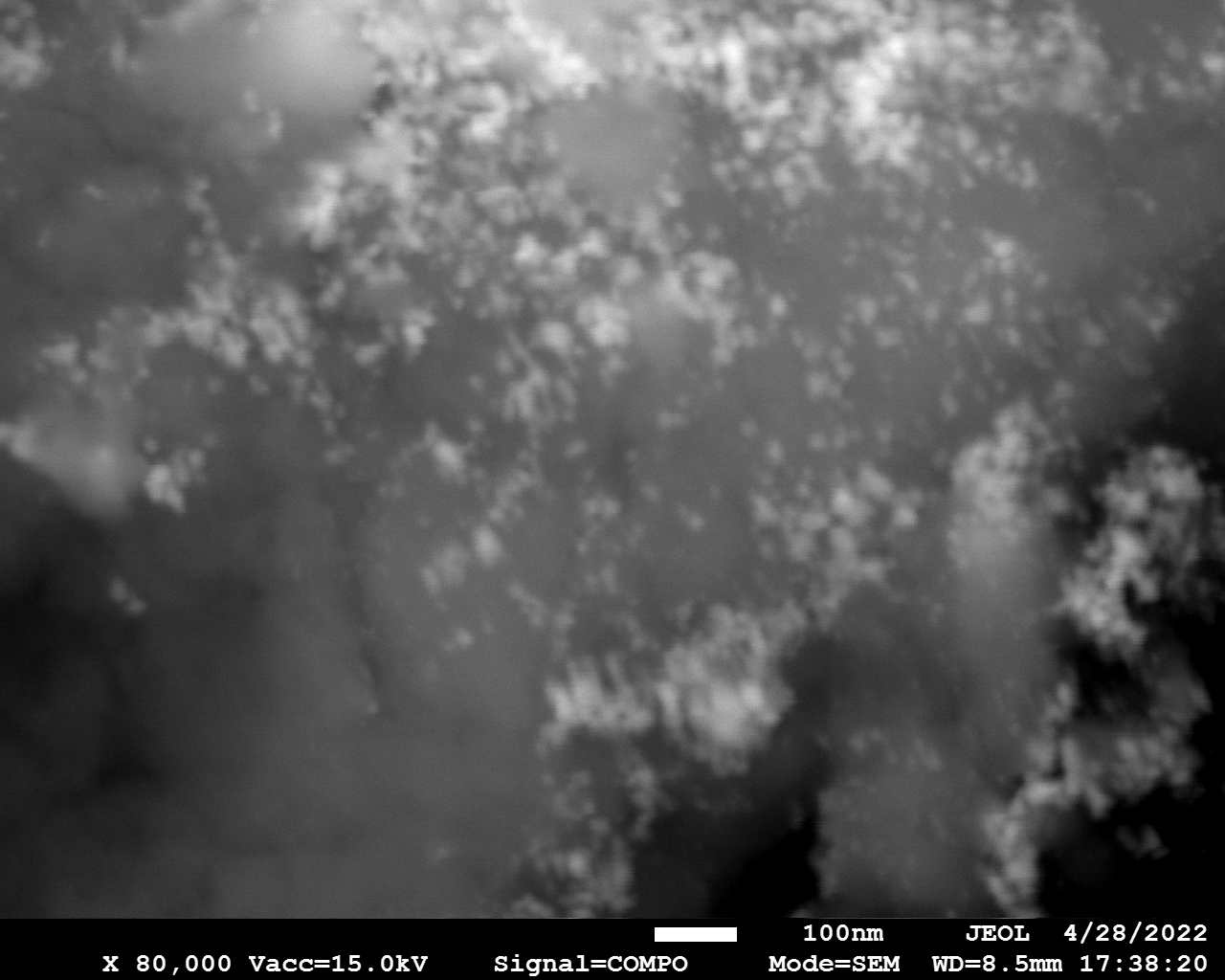
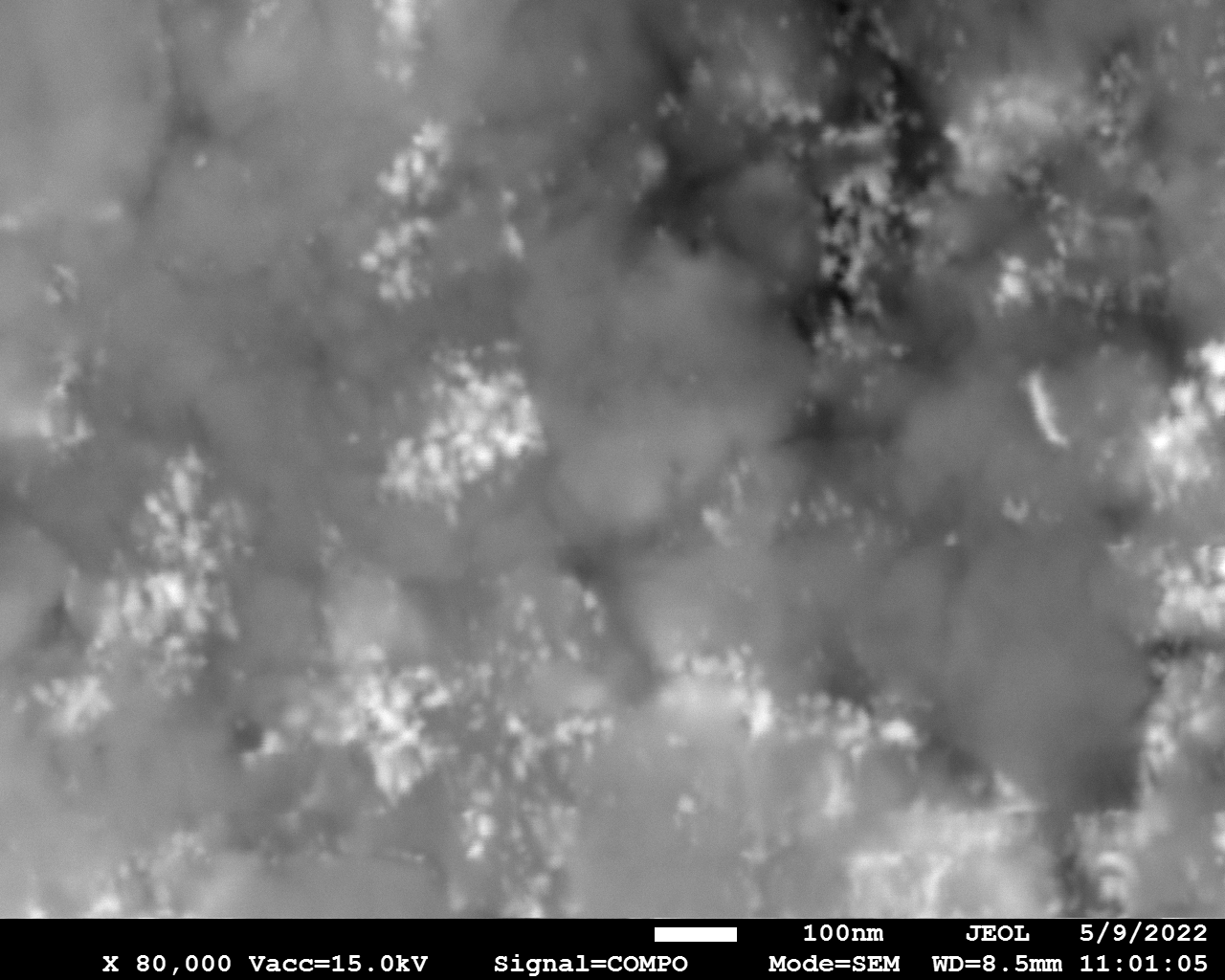
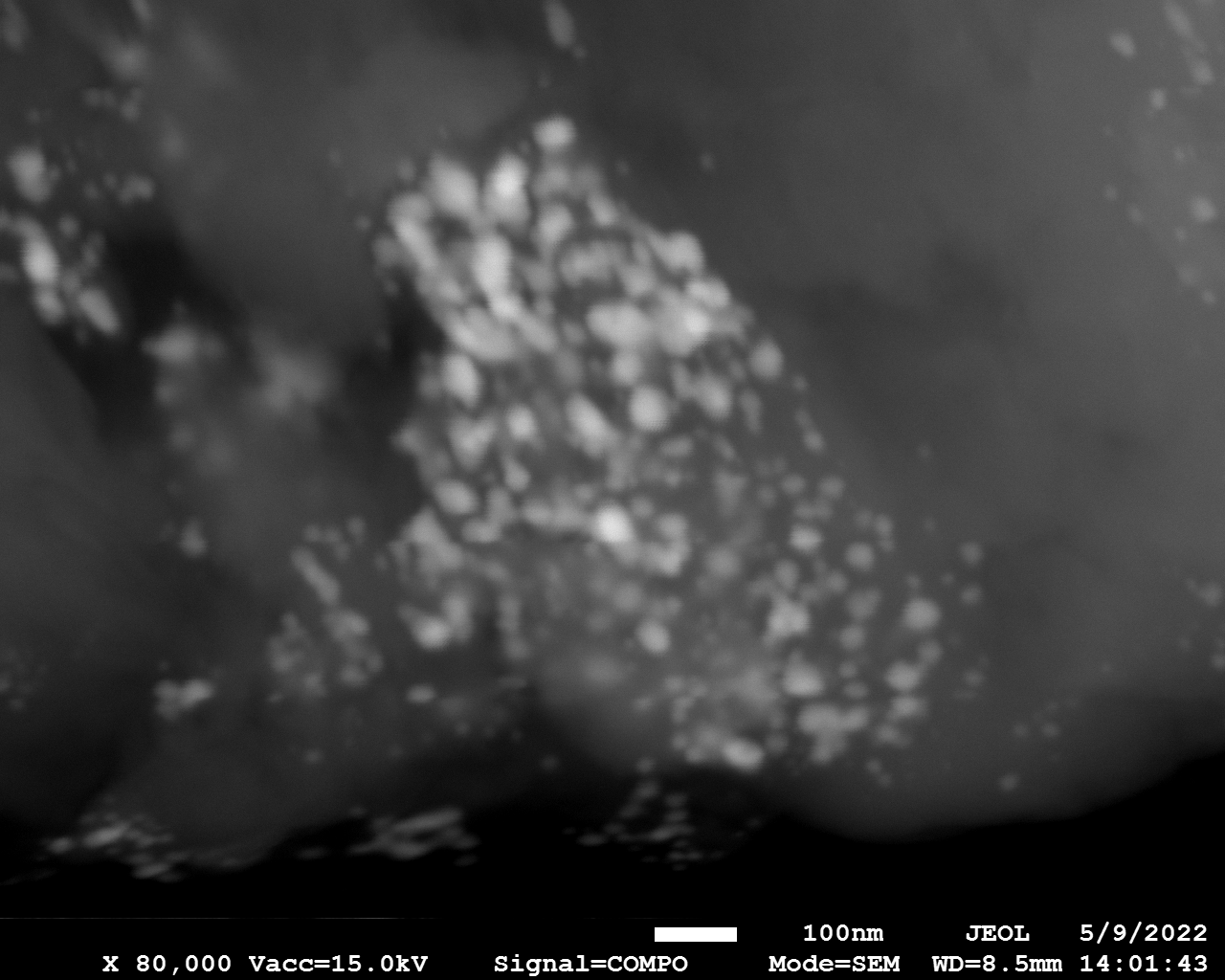
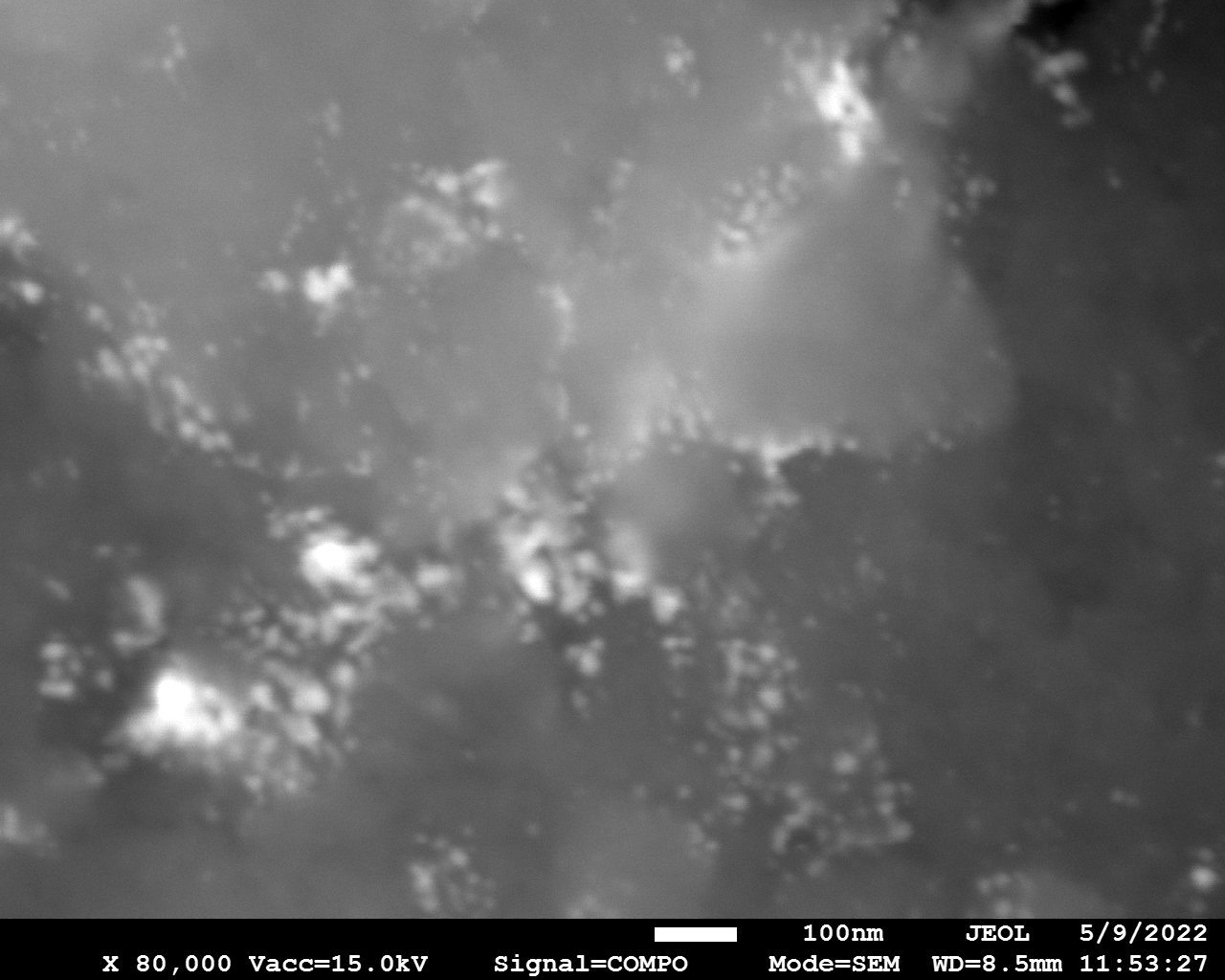
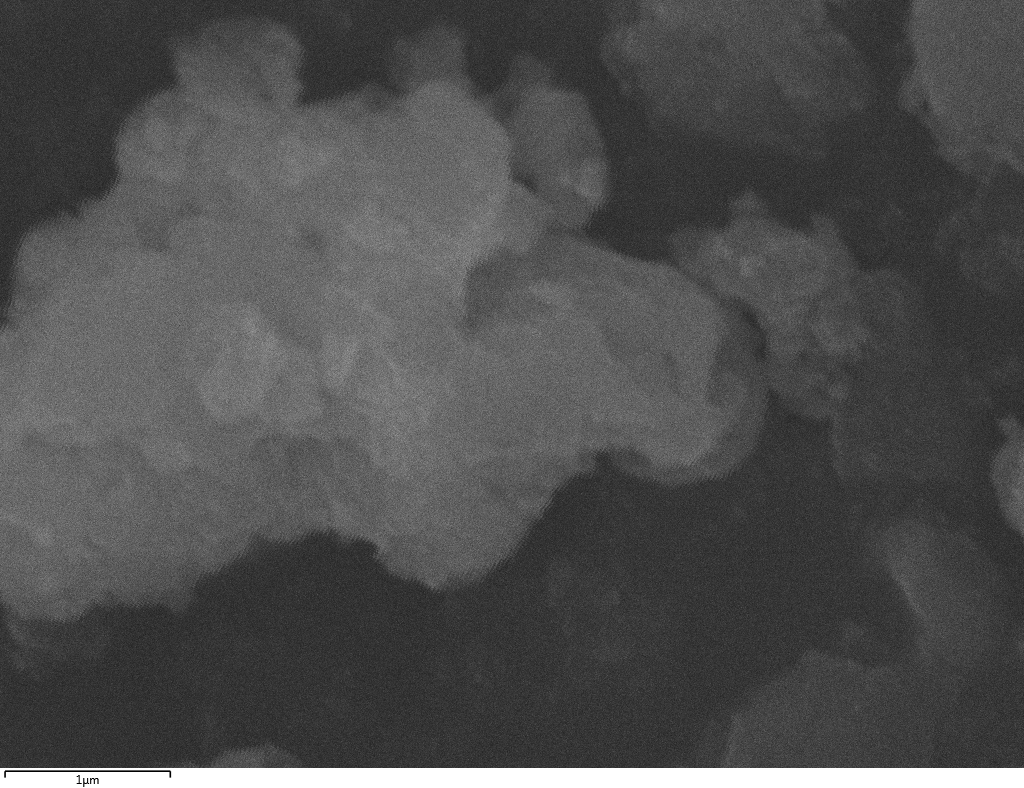
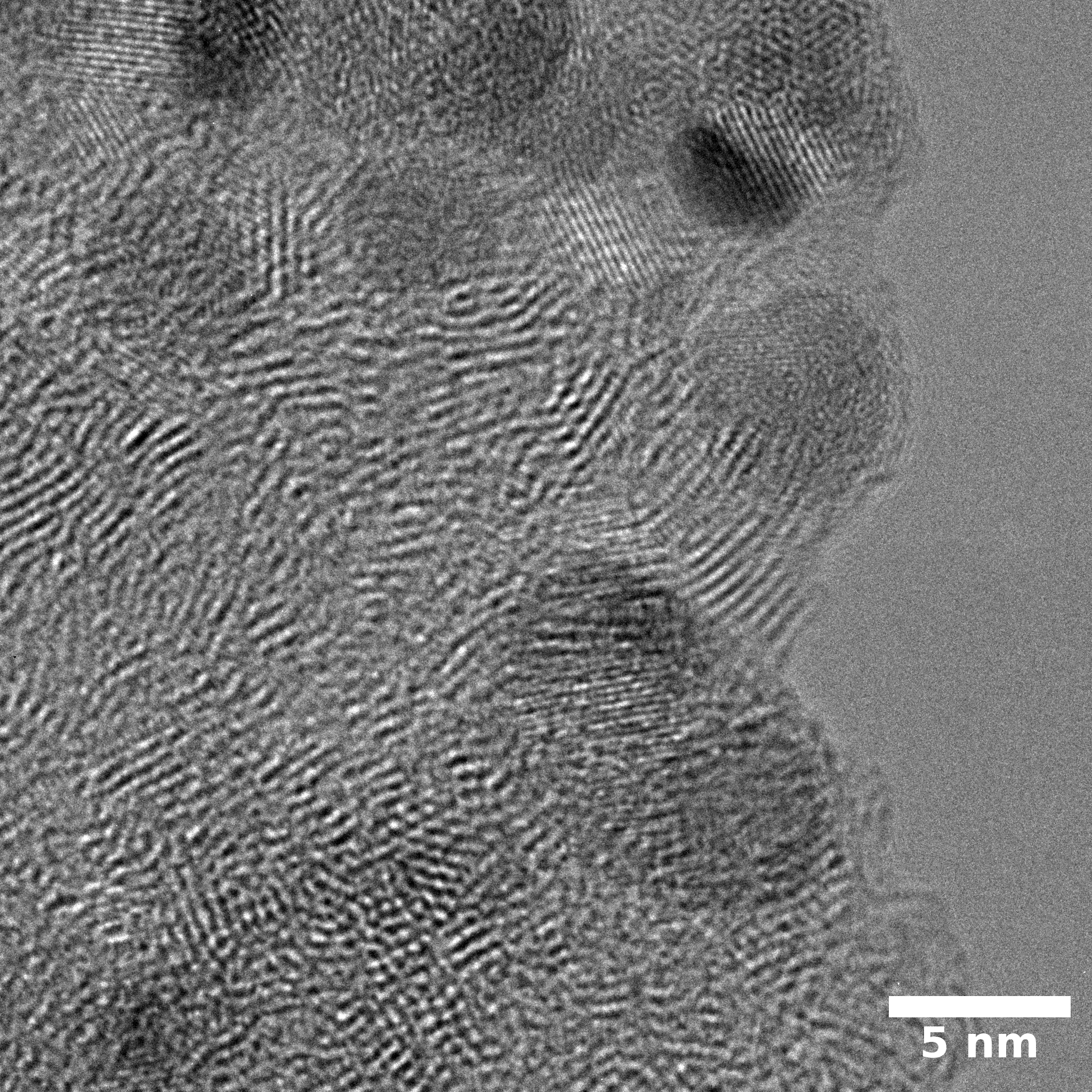
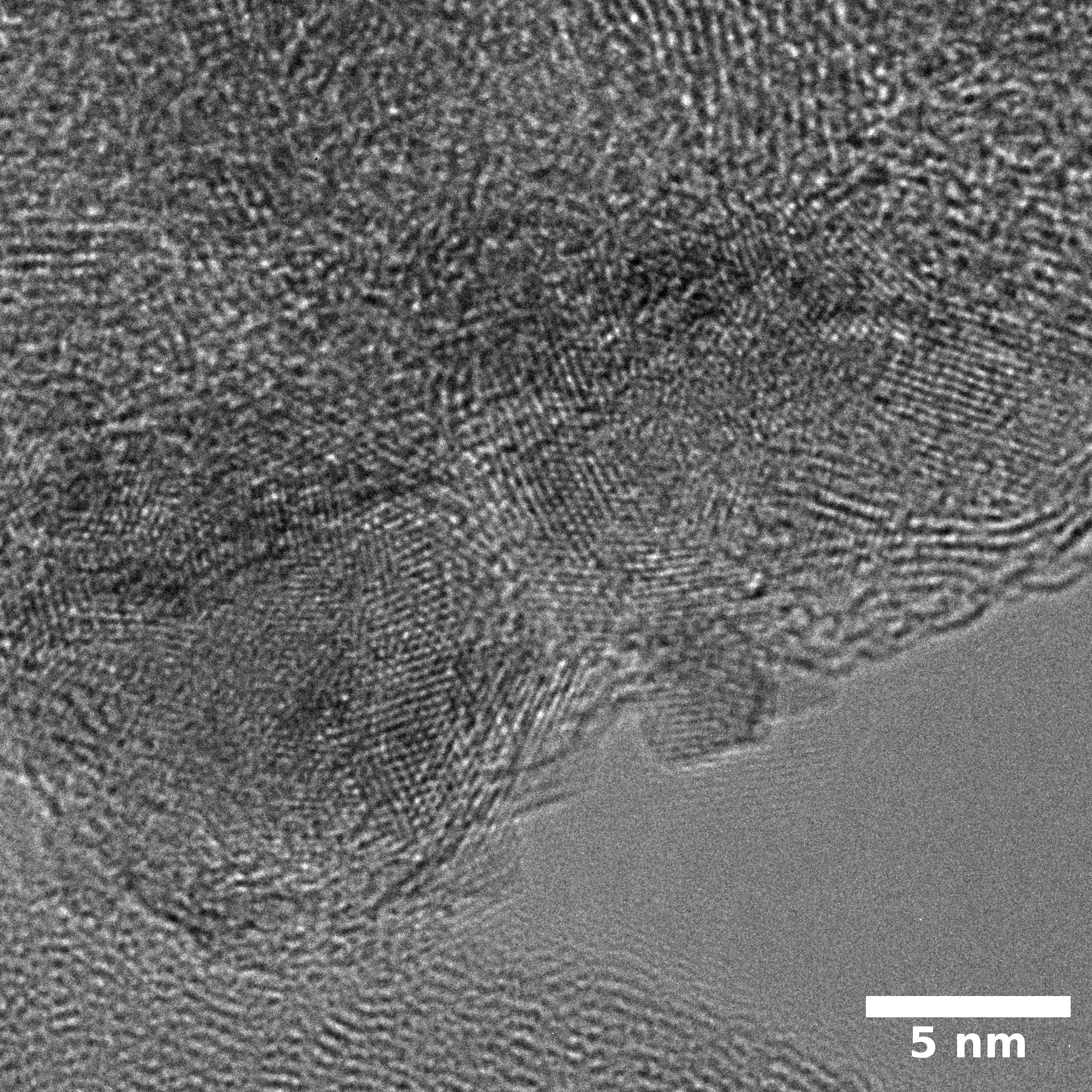
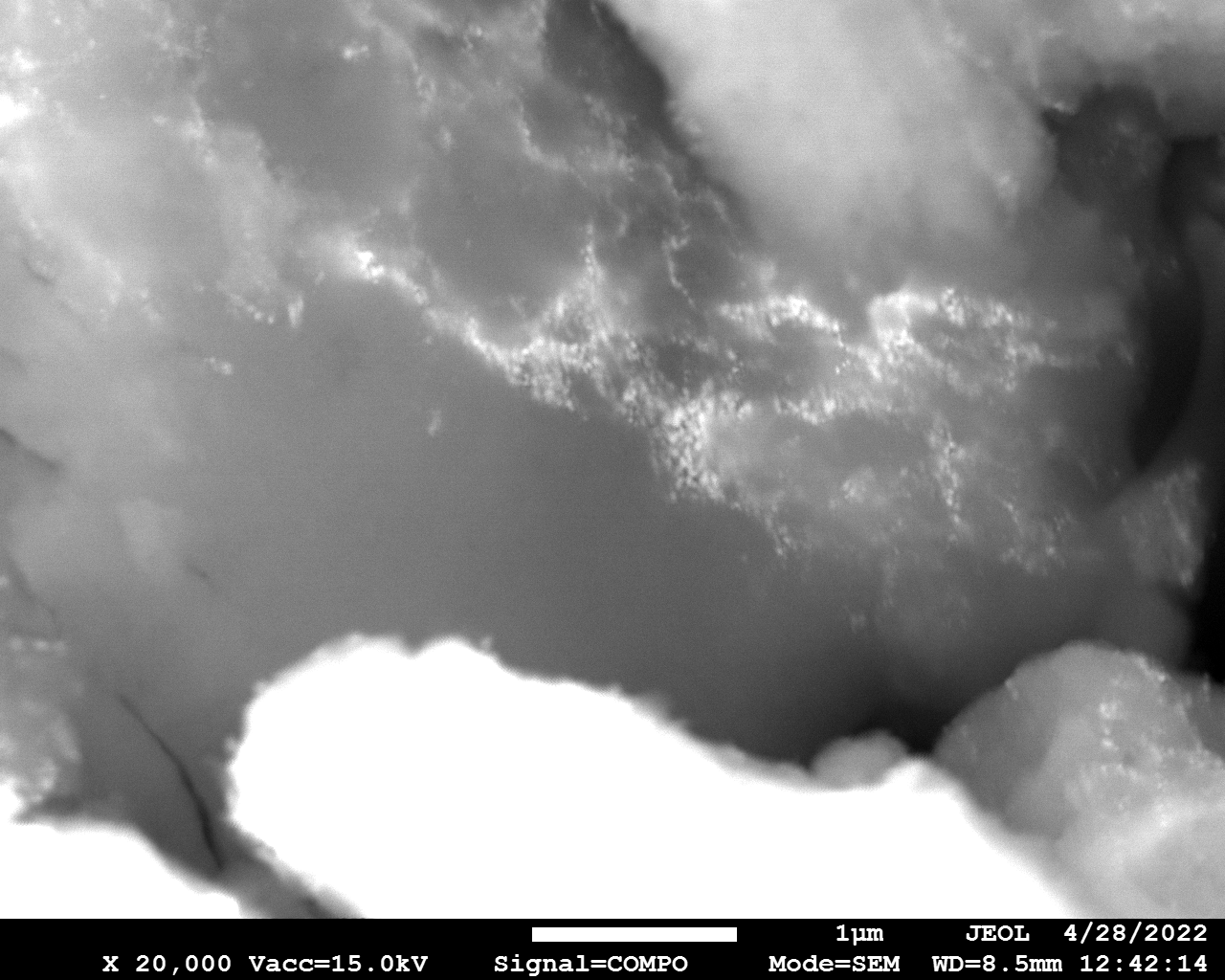
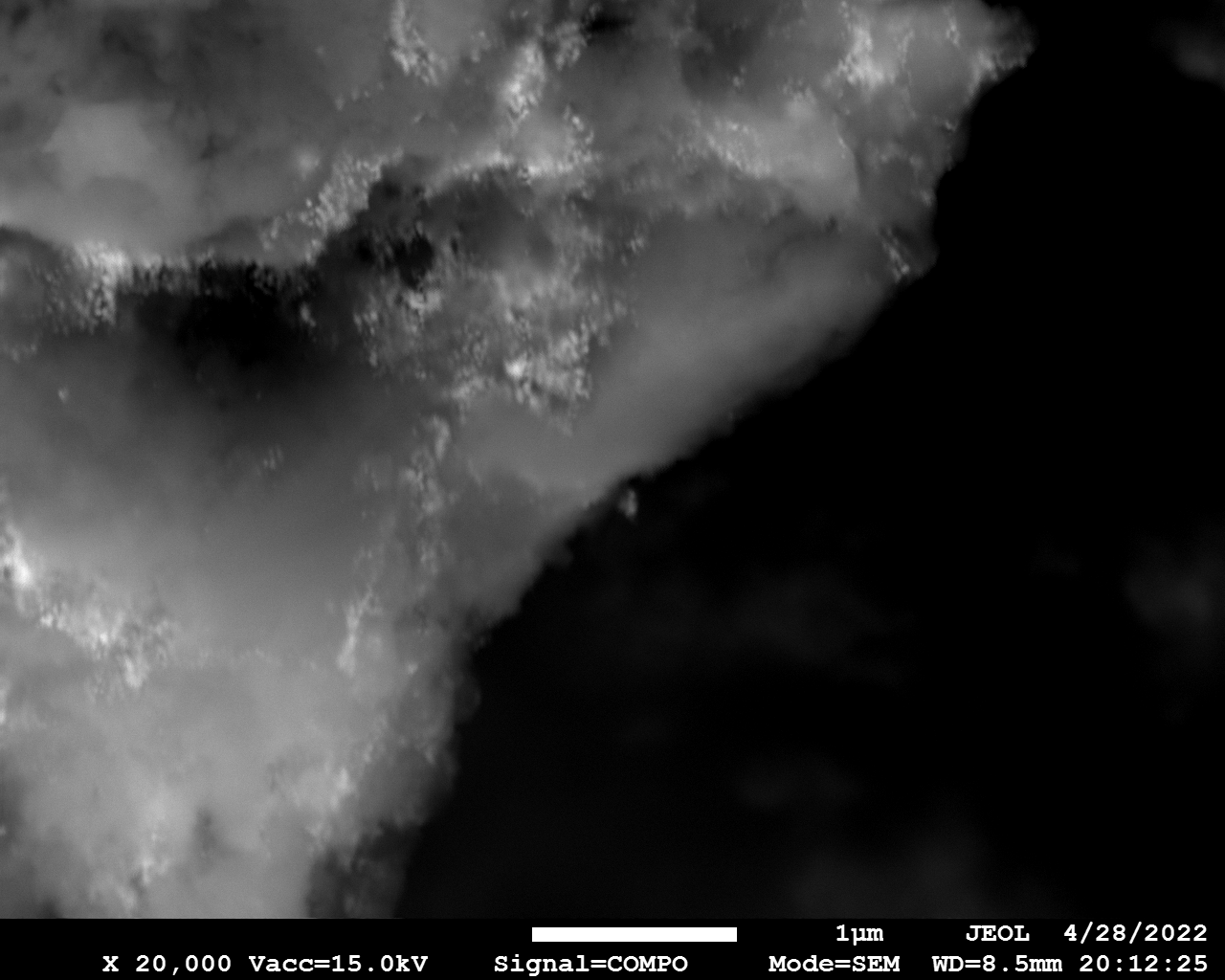
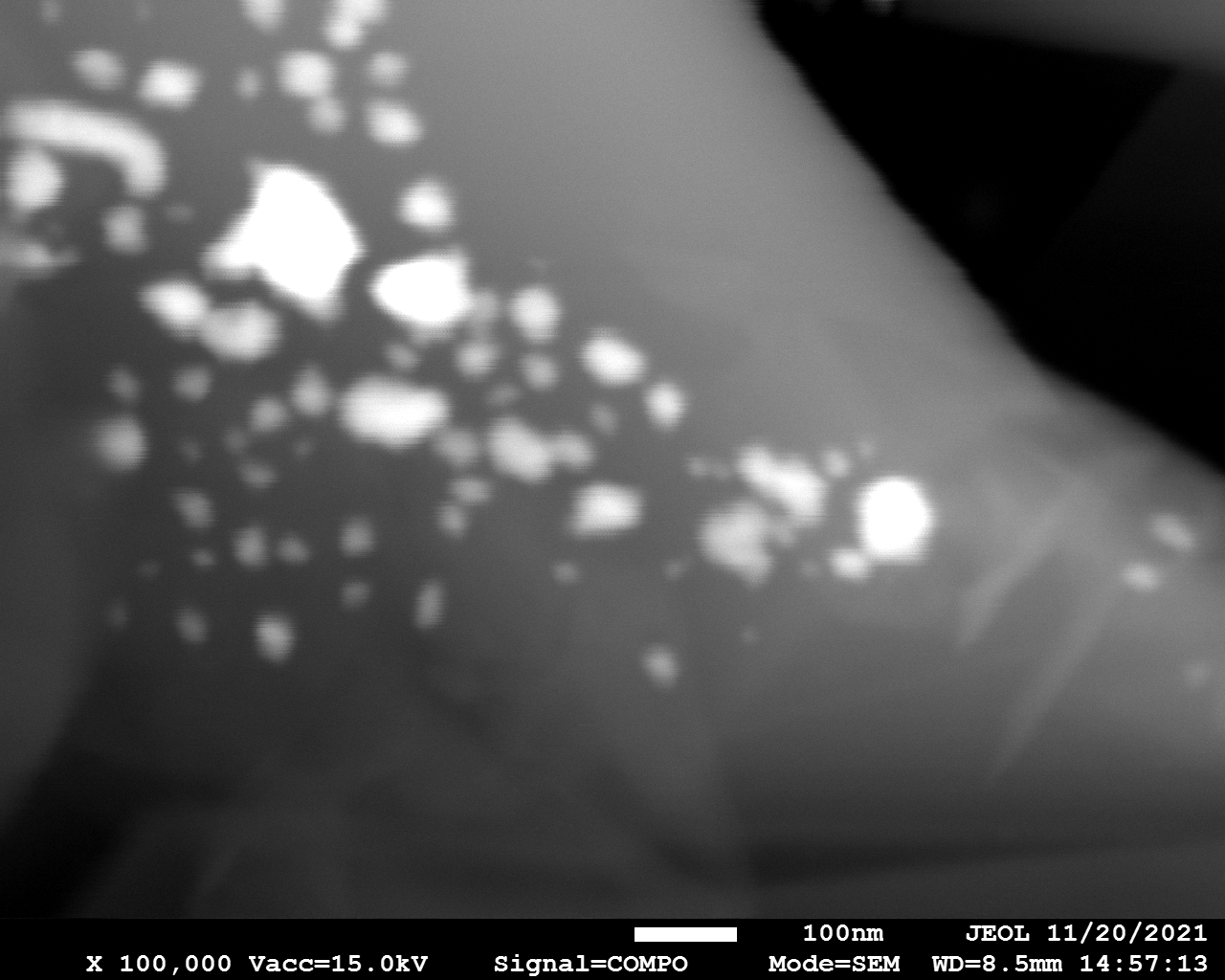
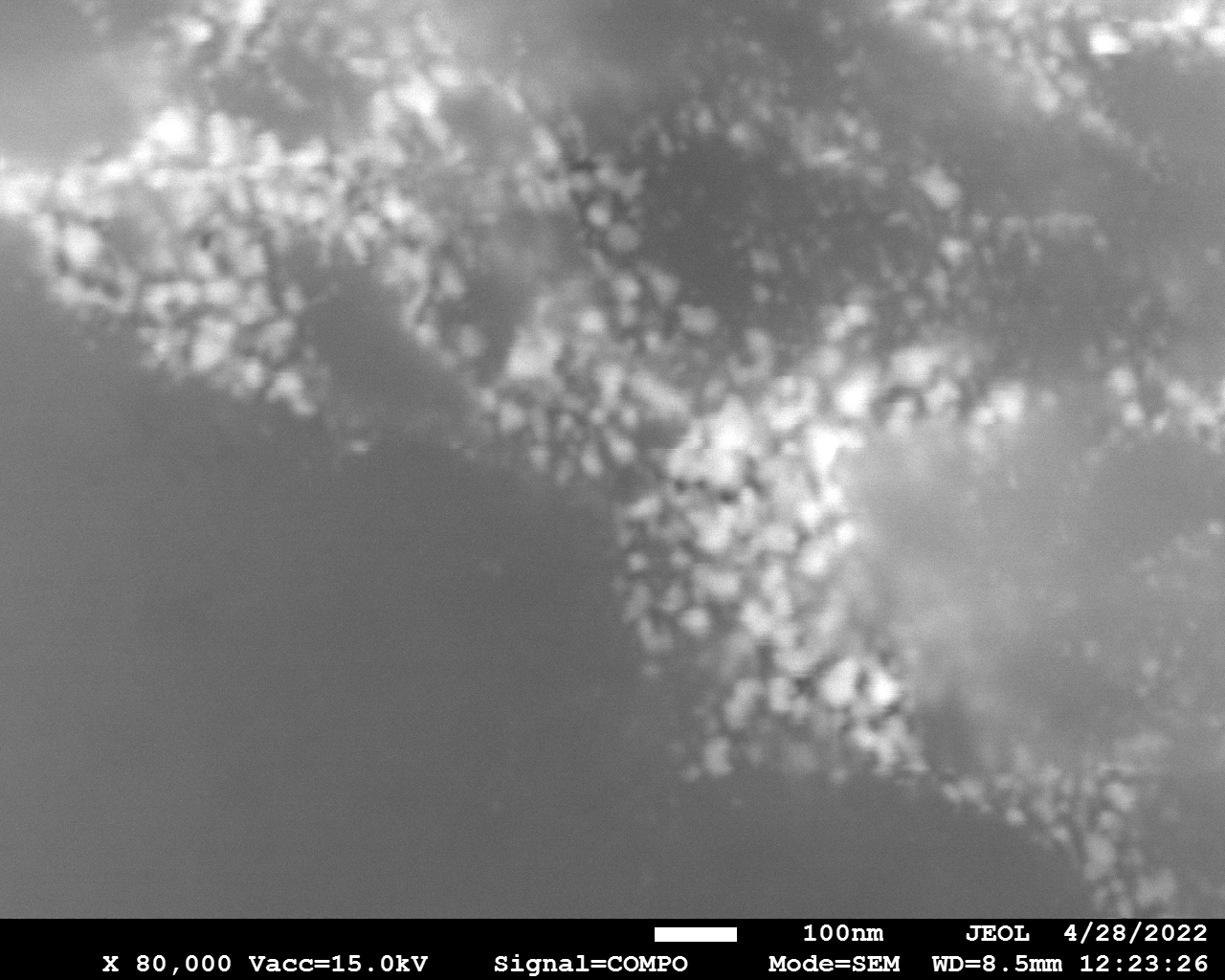
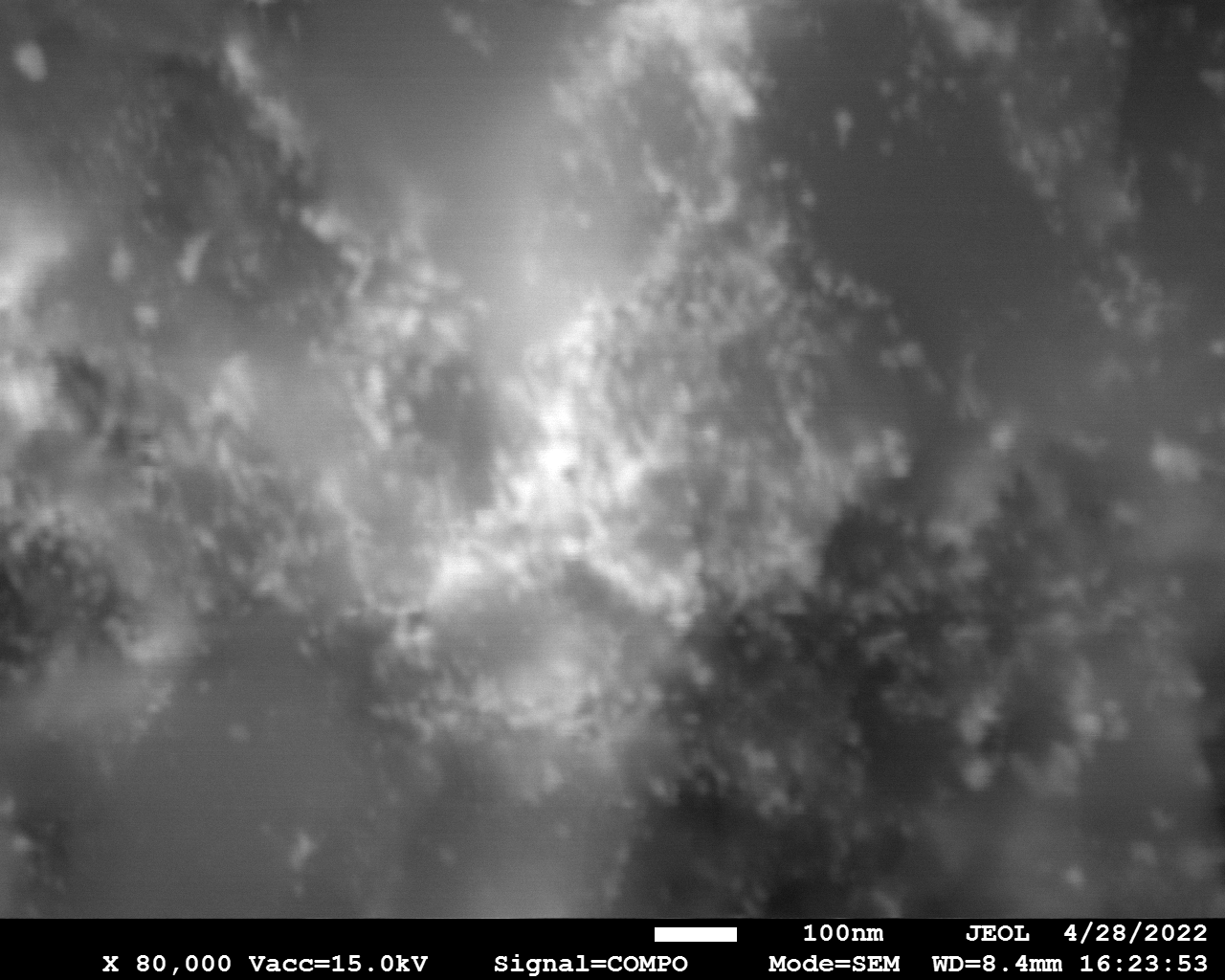
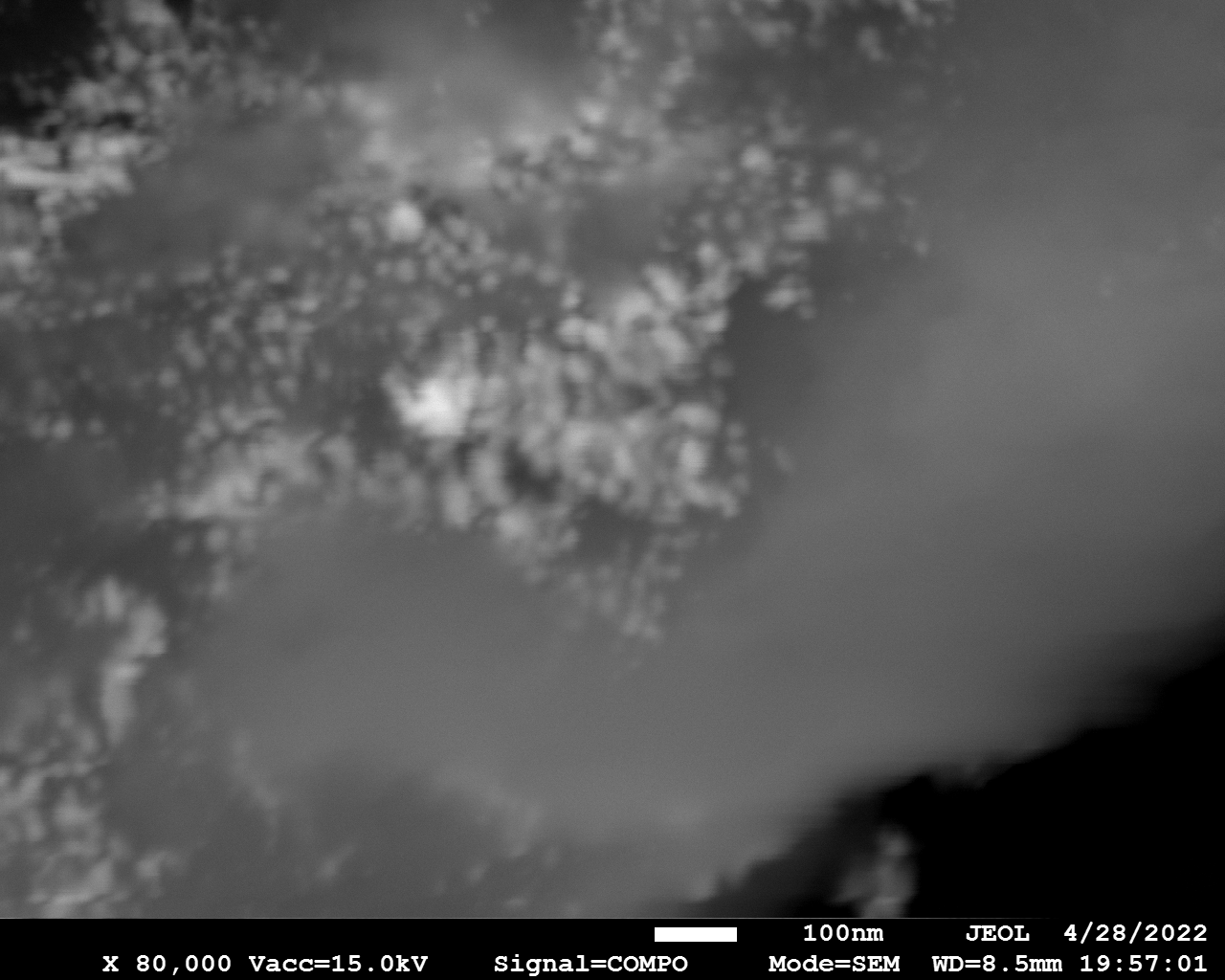
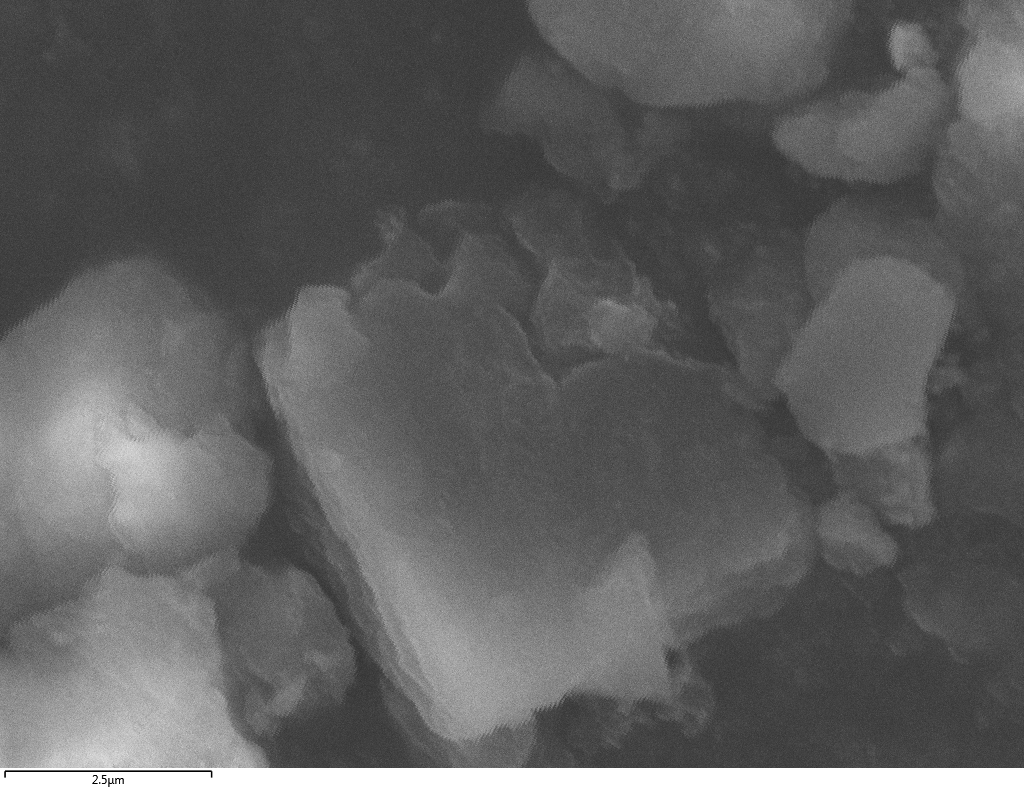
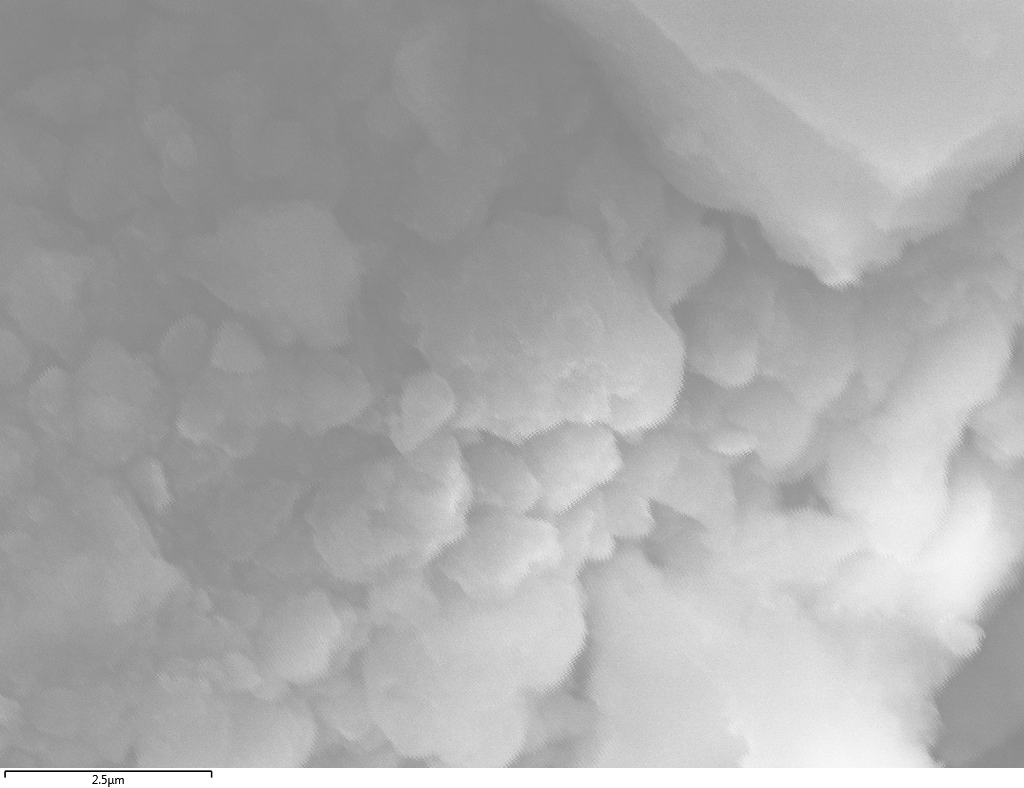
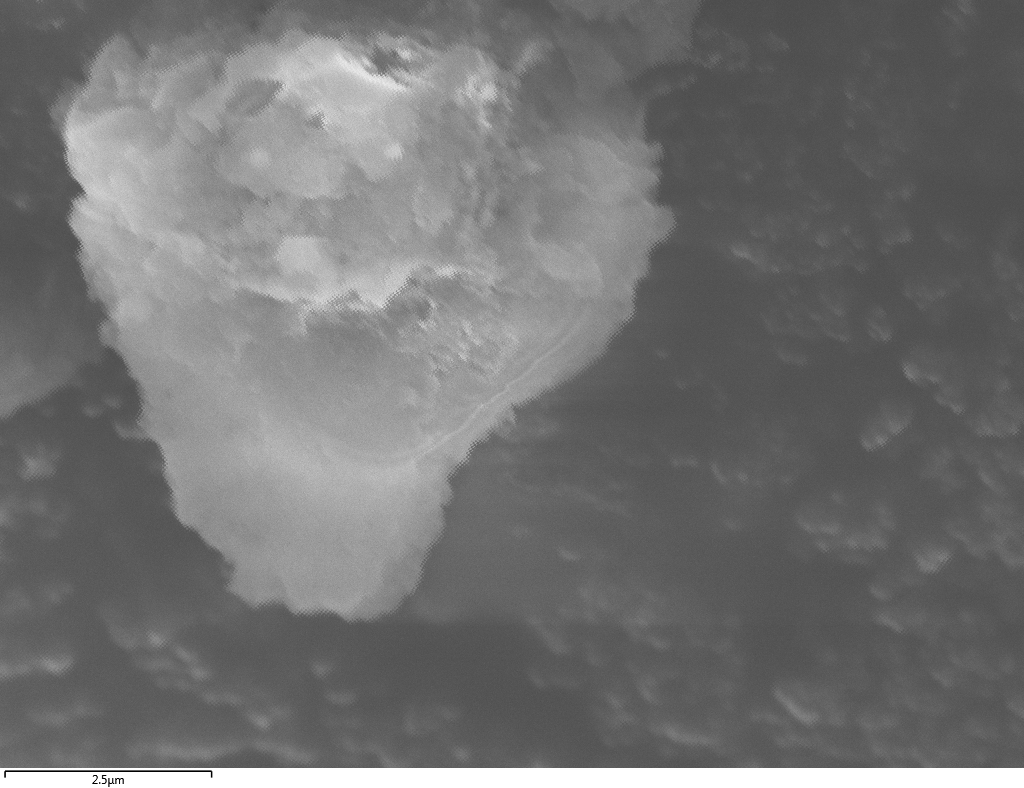
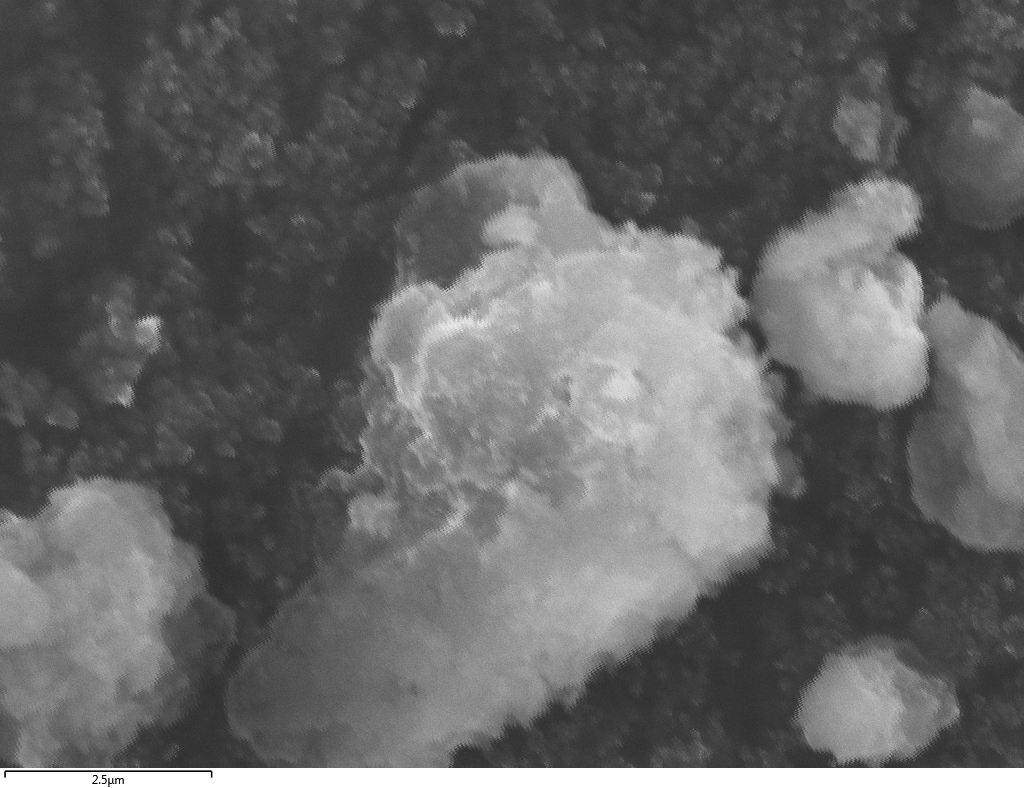
SEM
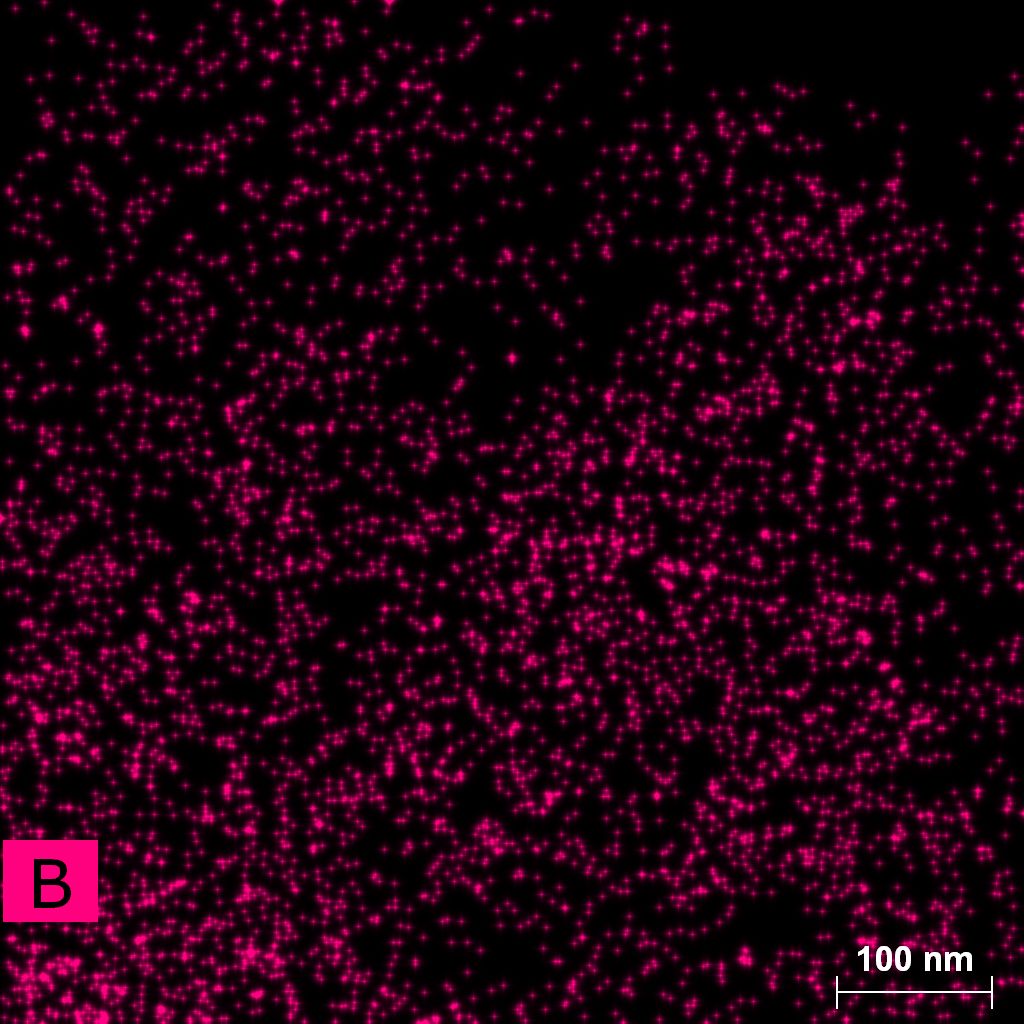
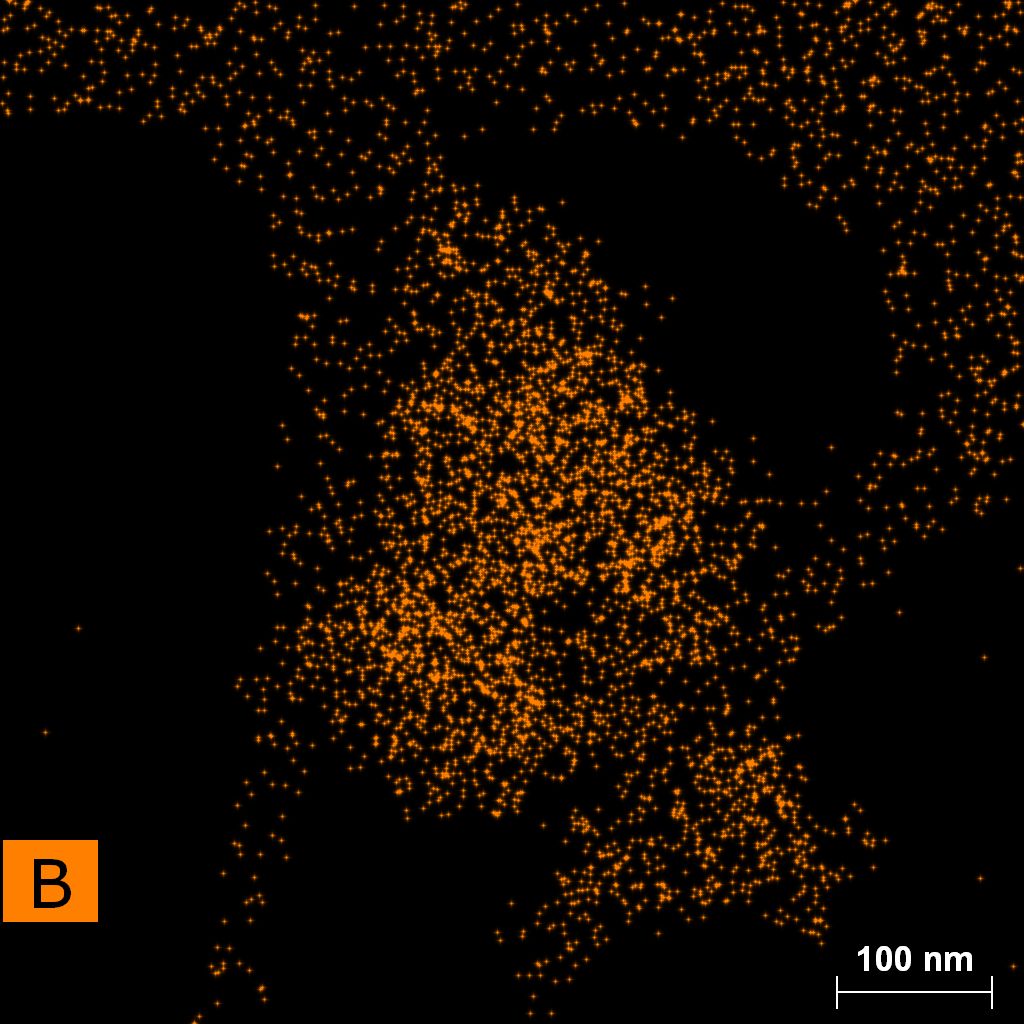
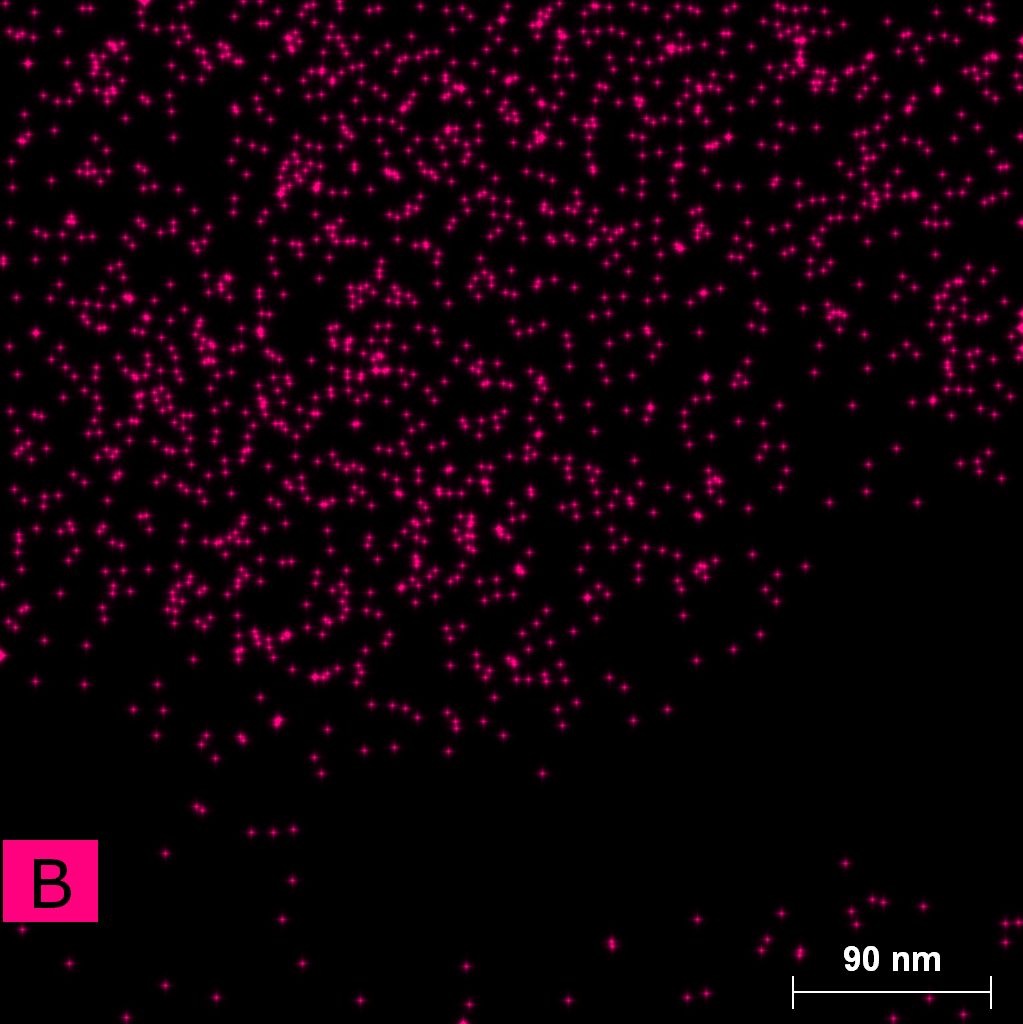
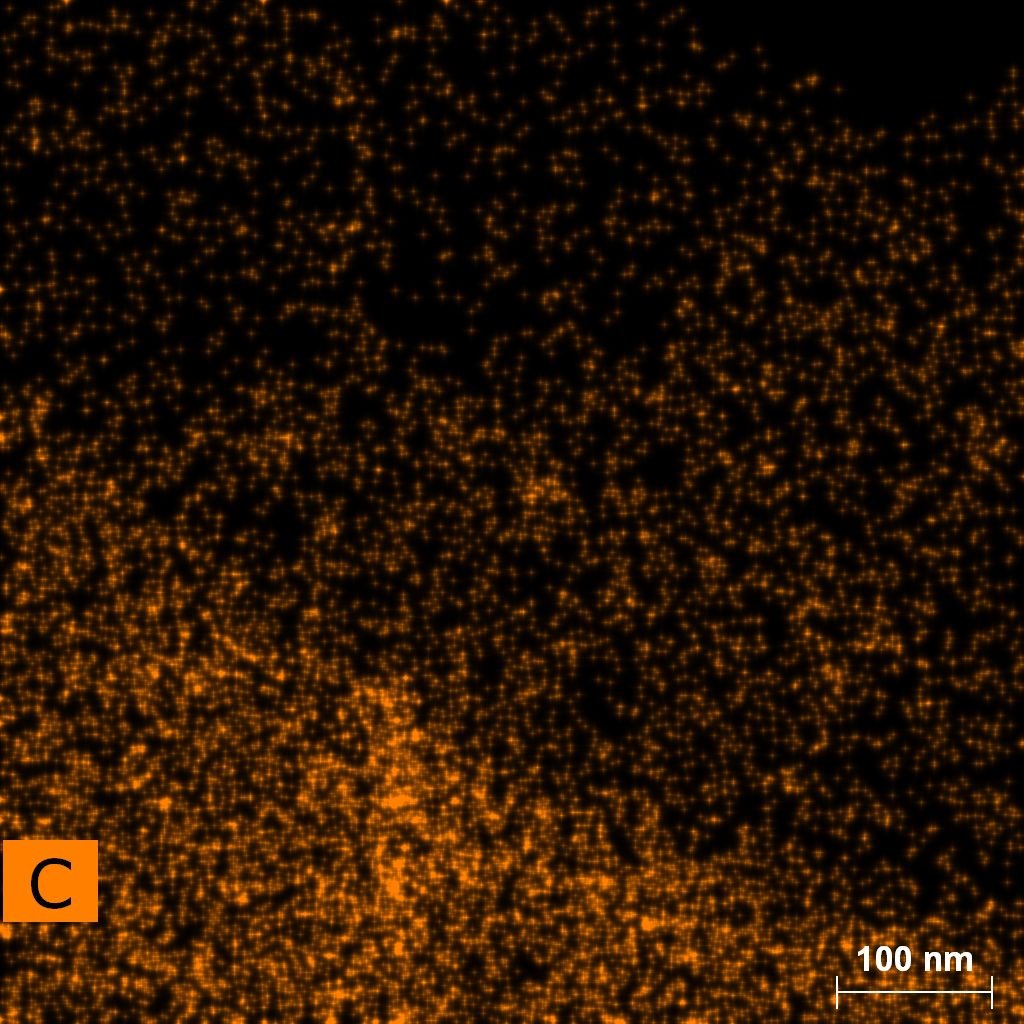
Samples consist of particles with size < 100 nm on the surface of h-BN. The increase of b/s ratio leads to decrease of particles size and their uniform distribution on the surface of hBN.
 |
 |
 |
 |
|
 |
 |
 |
 |
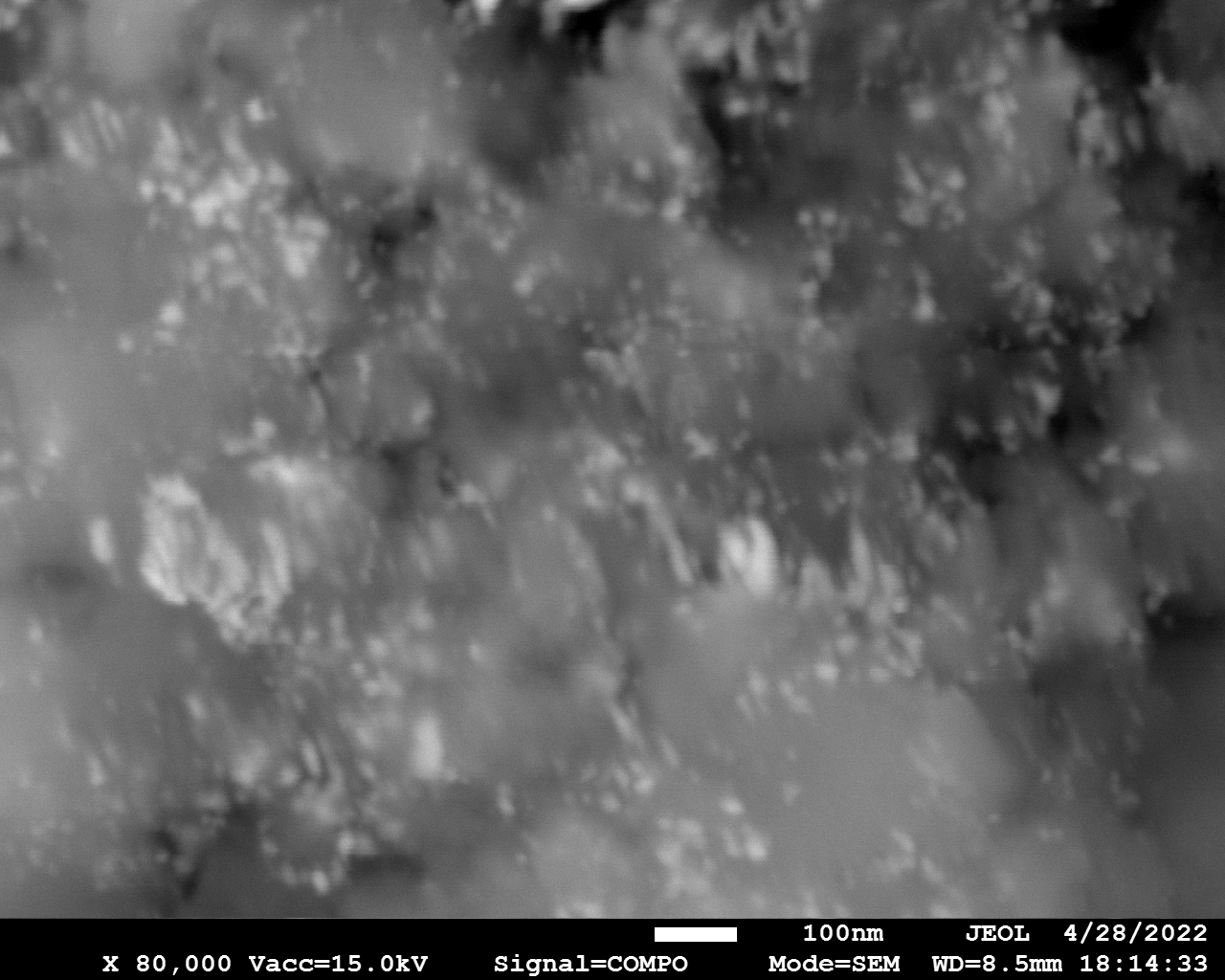 |
| dl109: no mill | dl121: b/s=22 | dl122: b/s=33 | dl126: b/s=44 | dl123: b/s=51 |
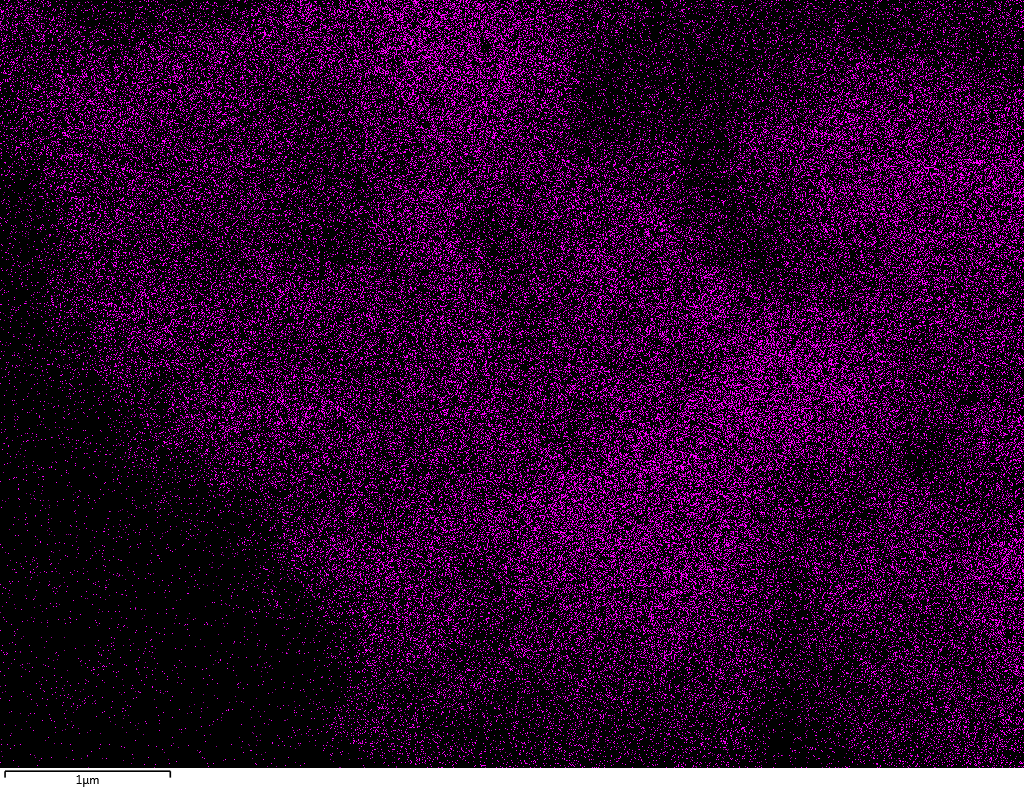
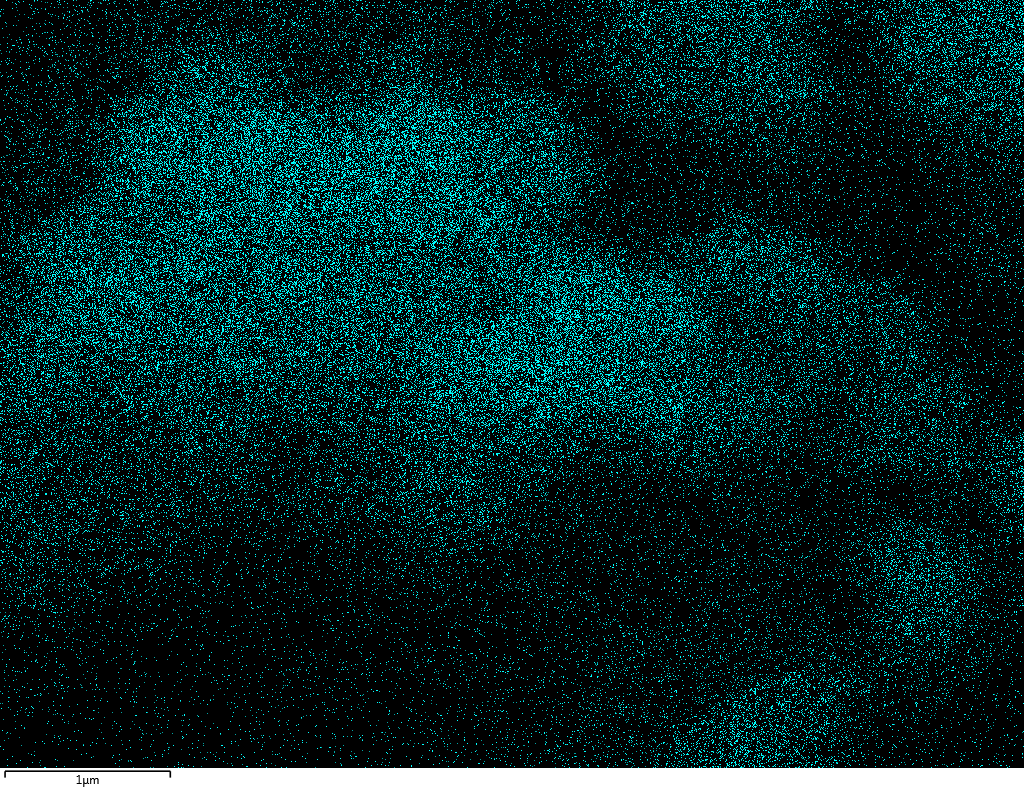
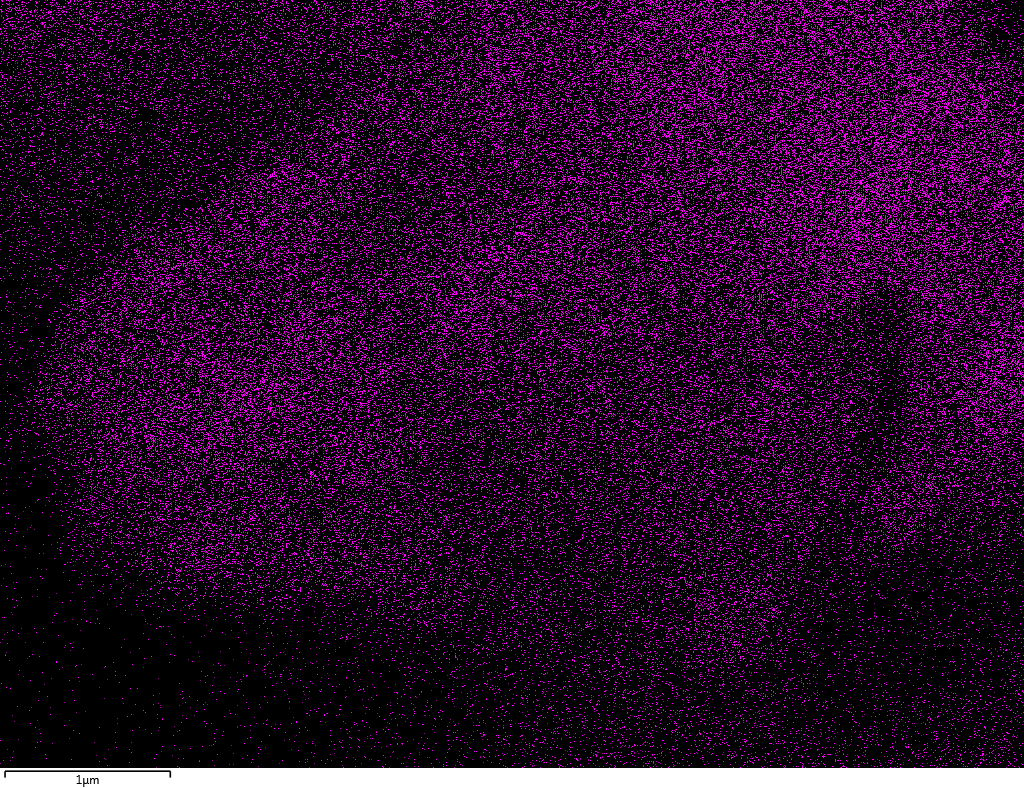
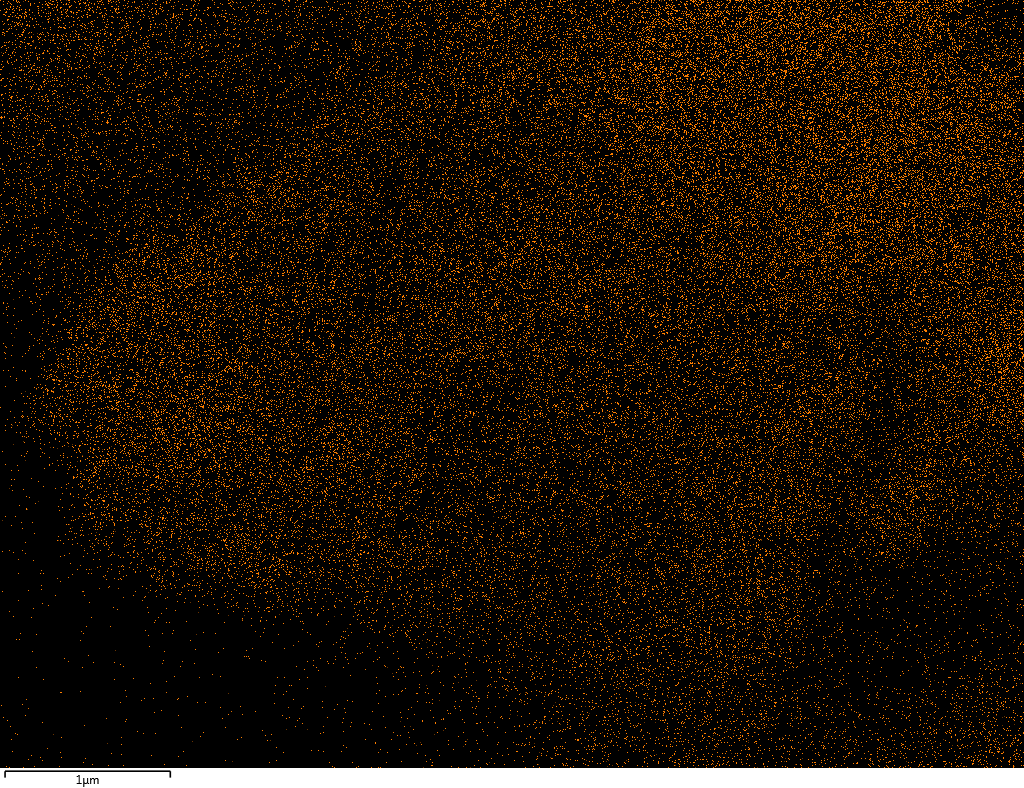
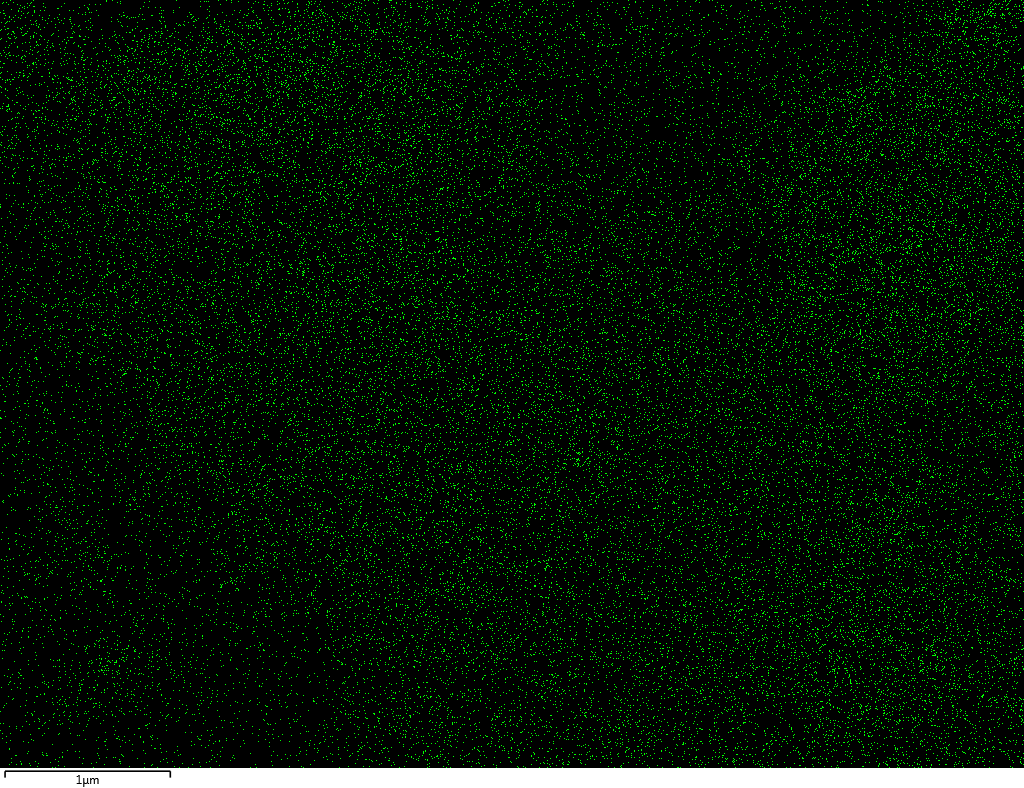
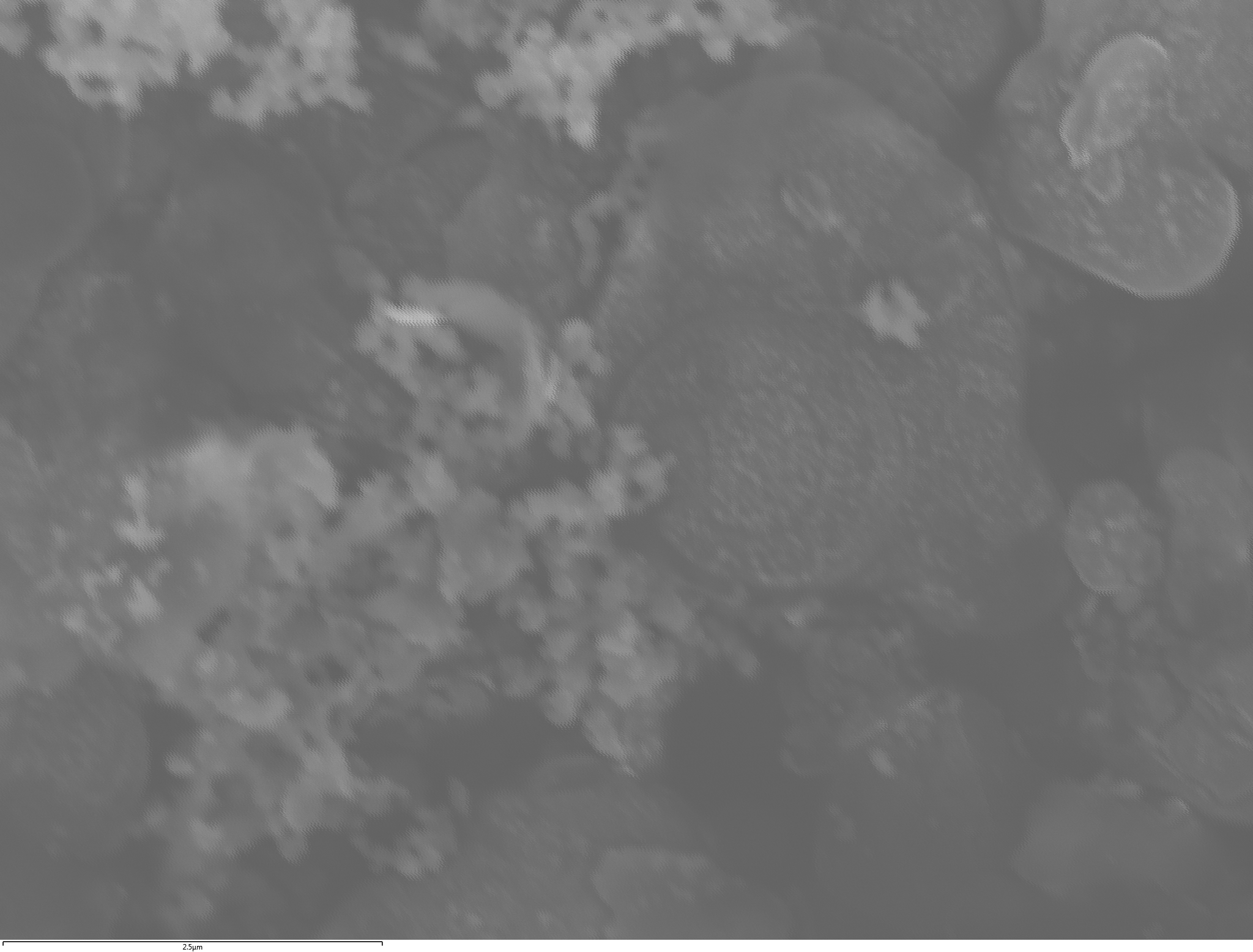
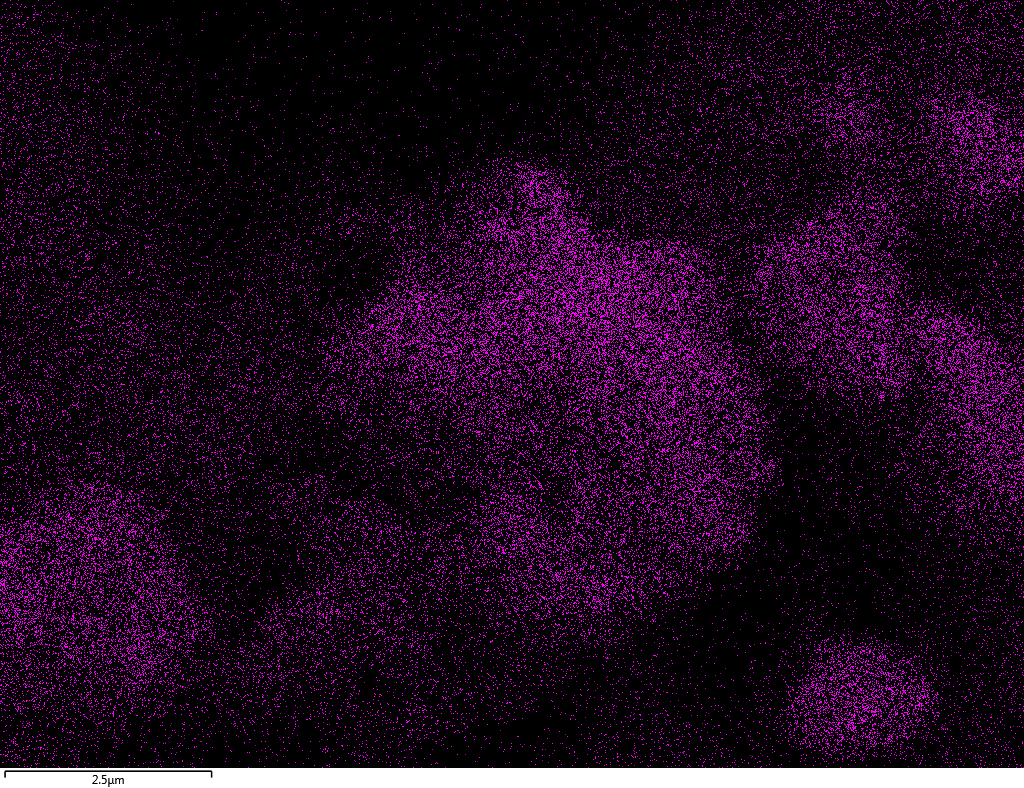
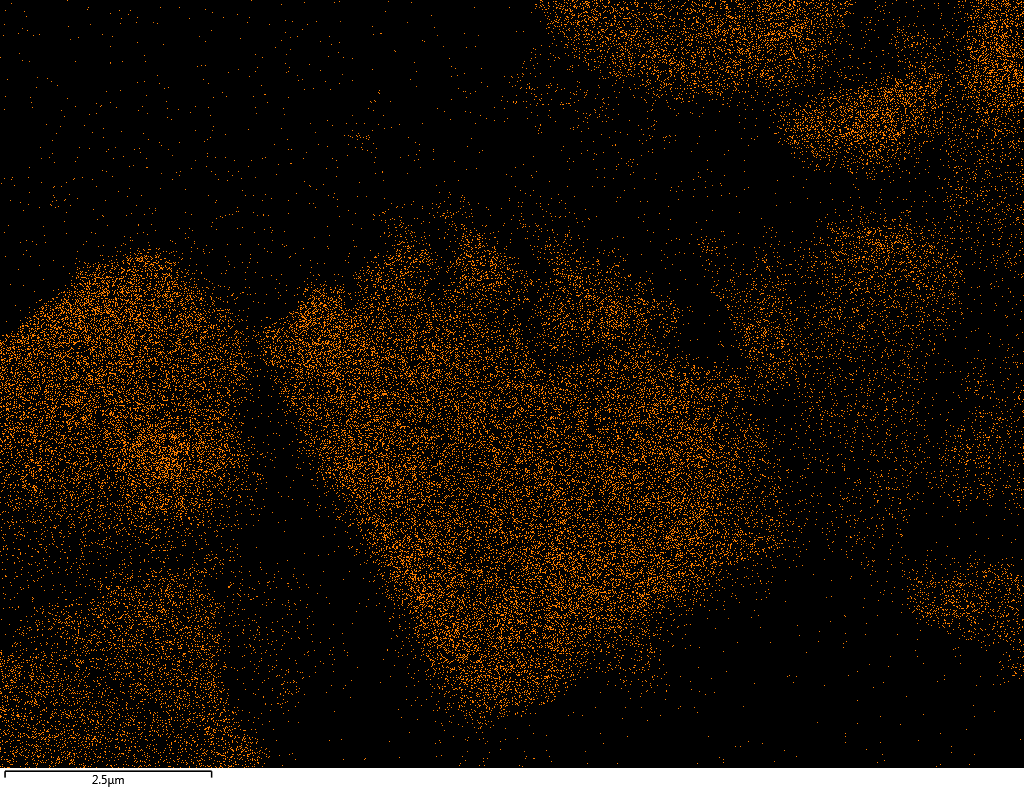
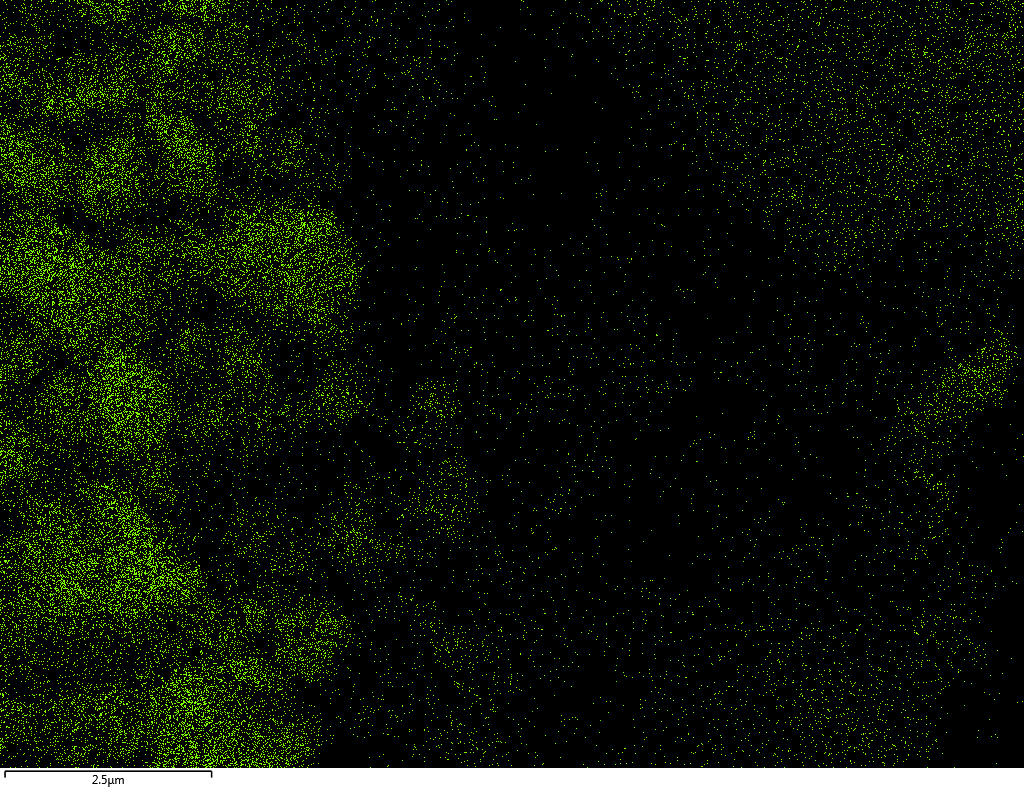
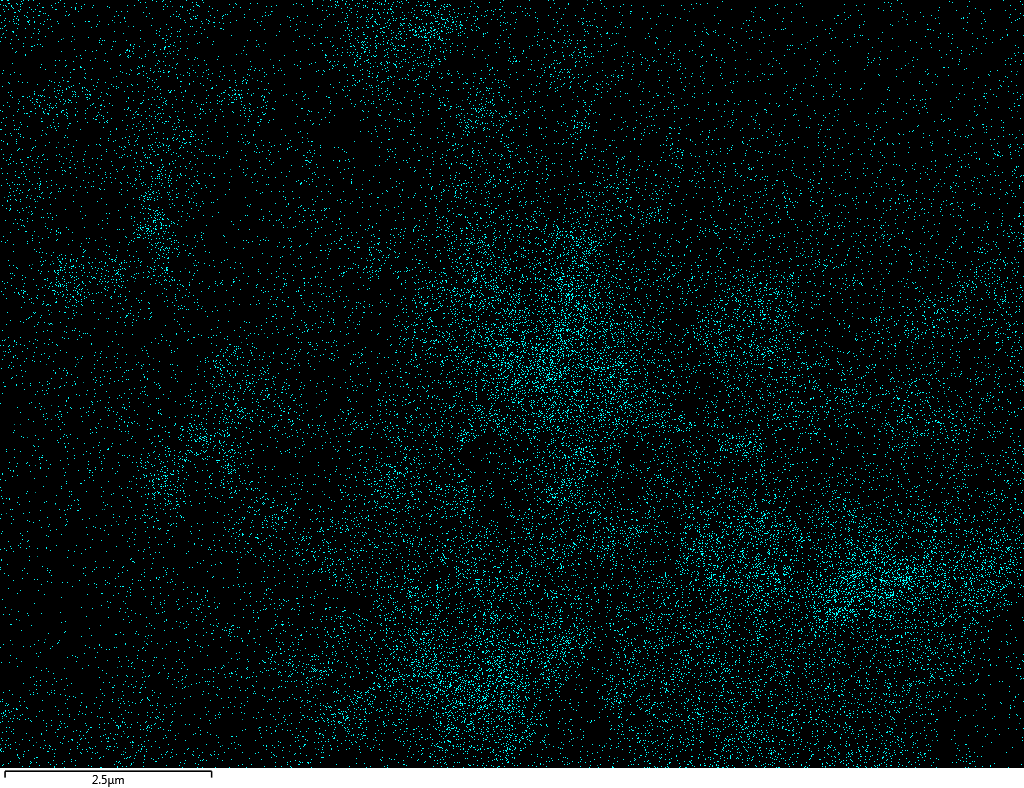
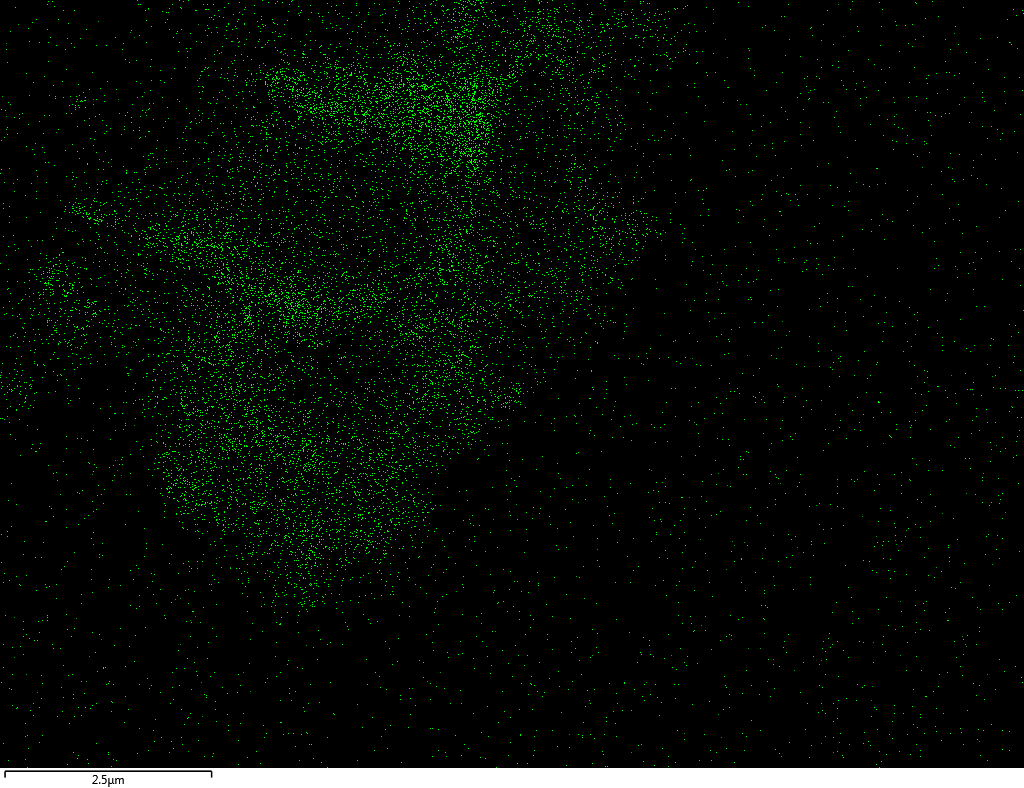
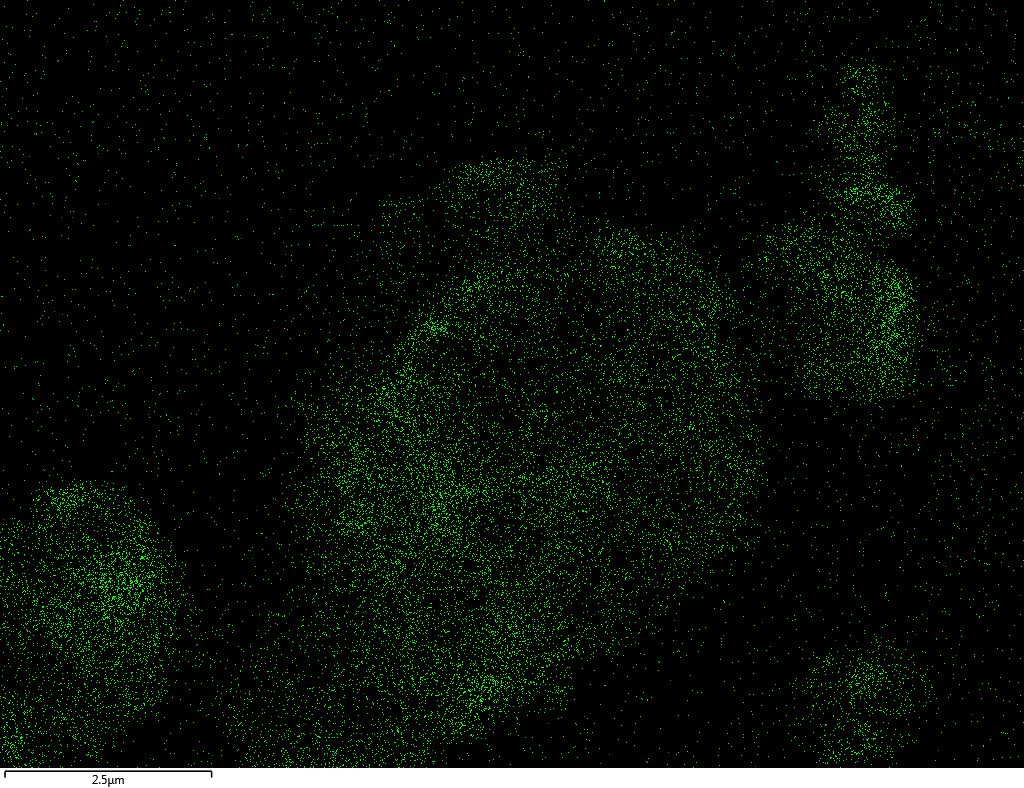
EDX
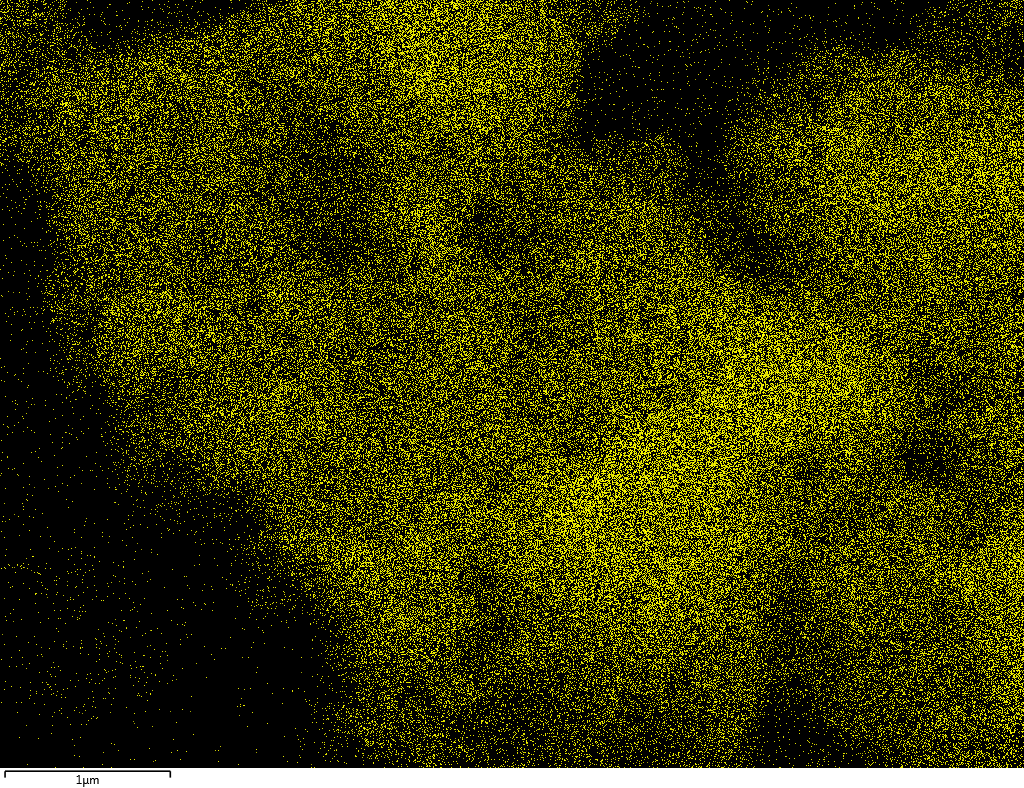
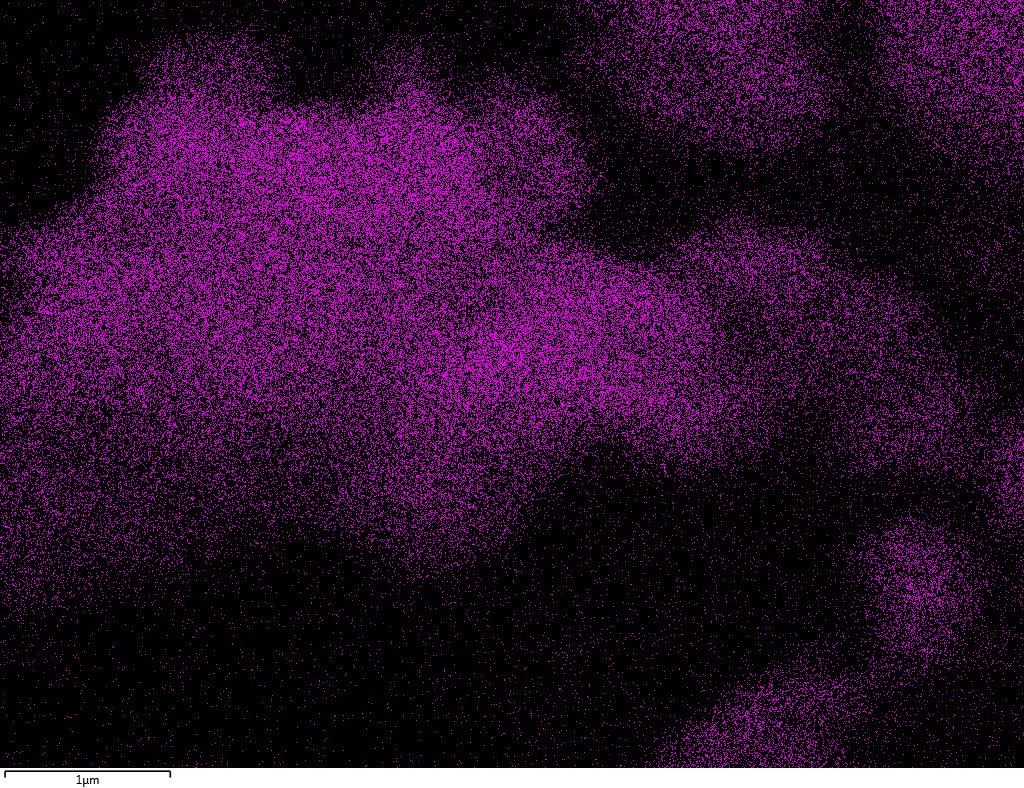
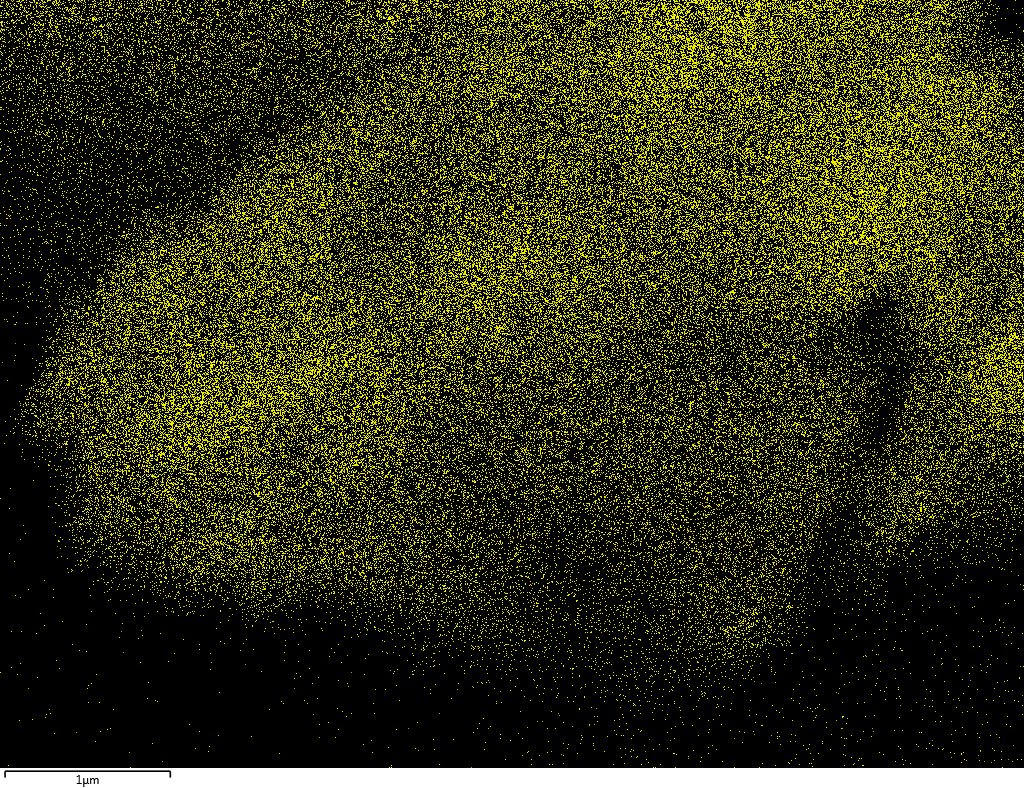
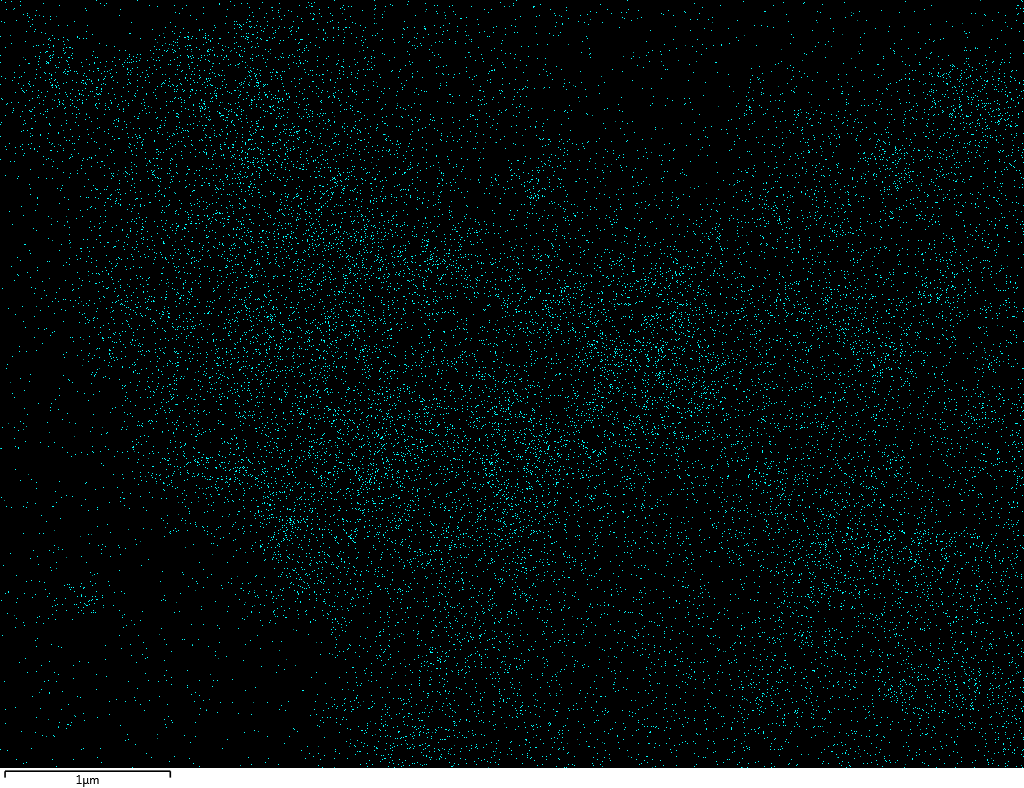
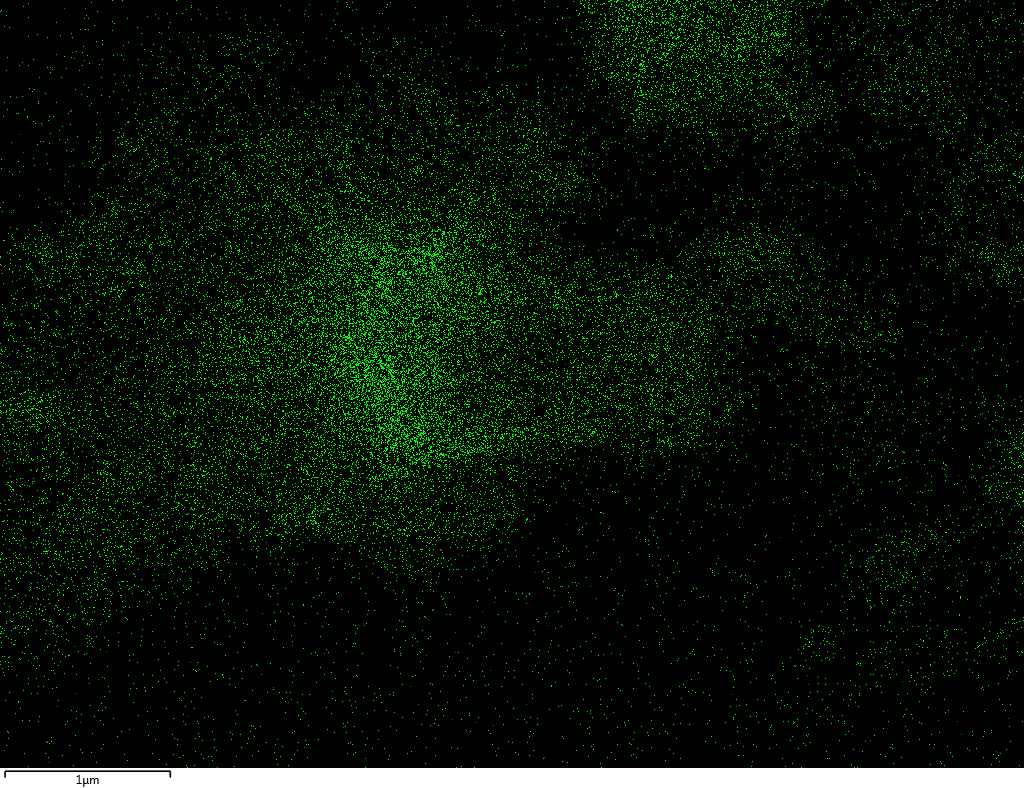
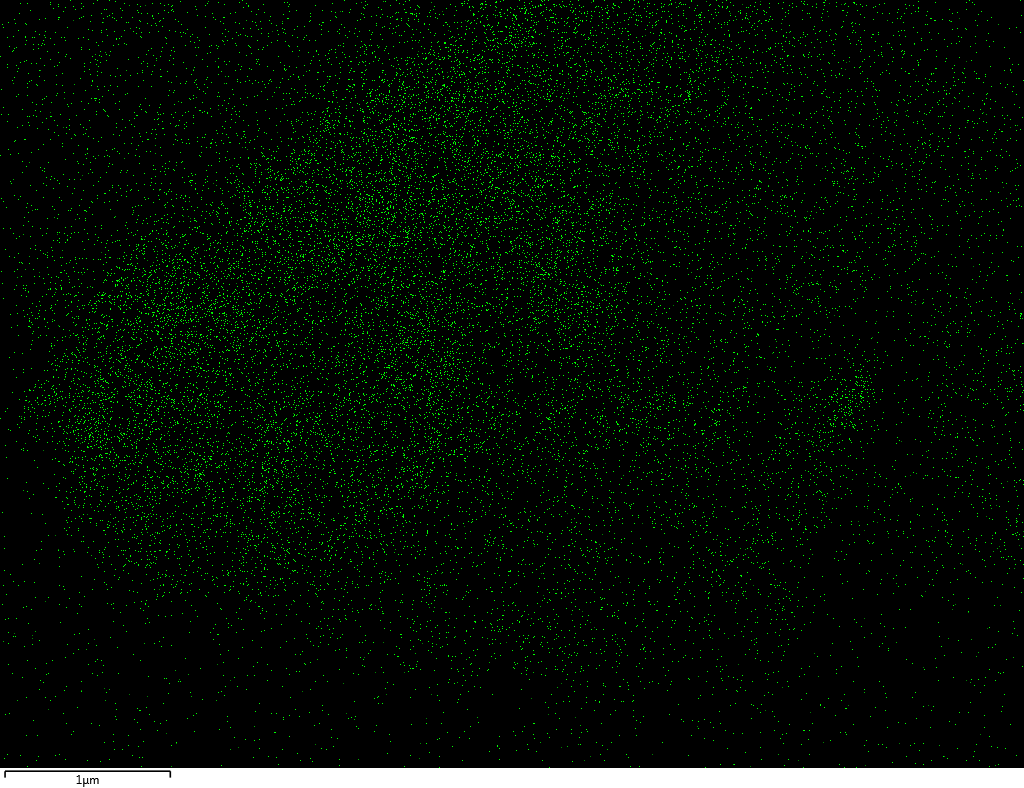
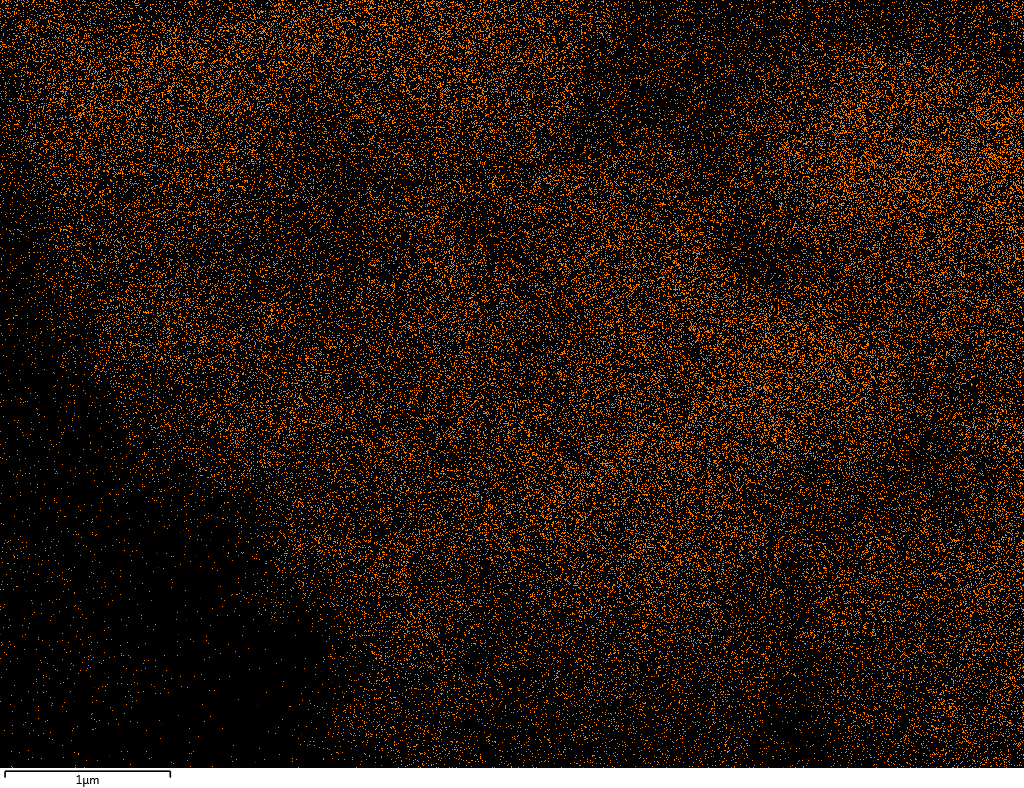
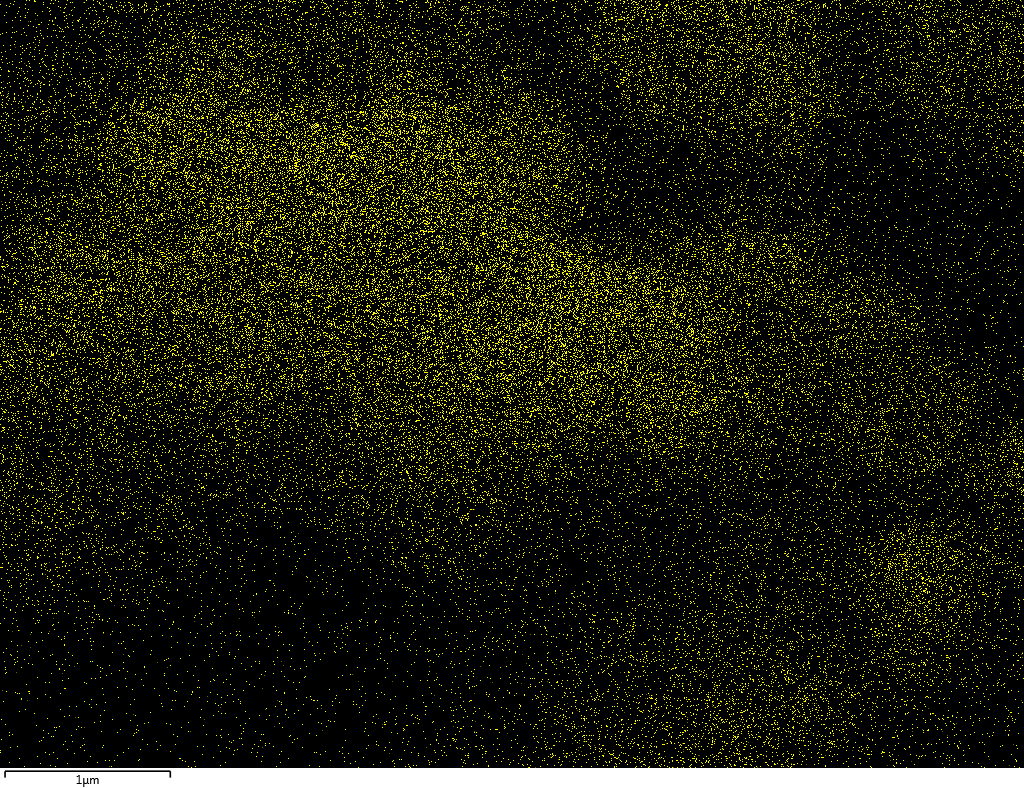
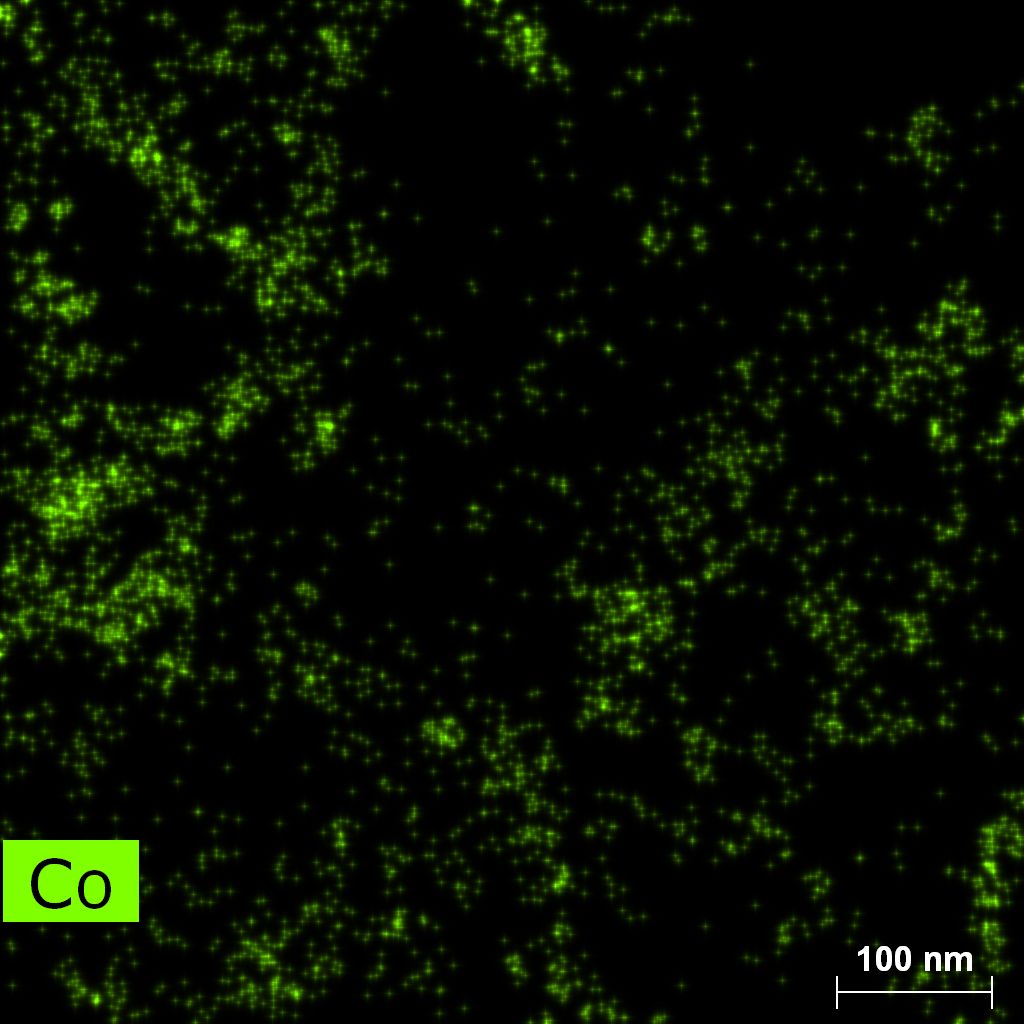
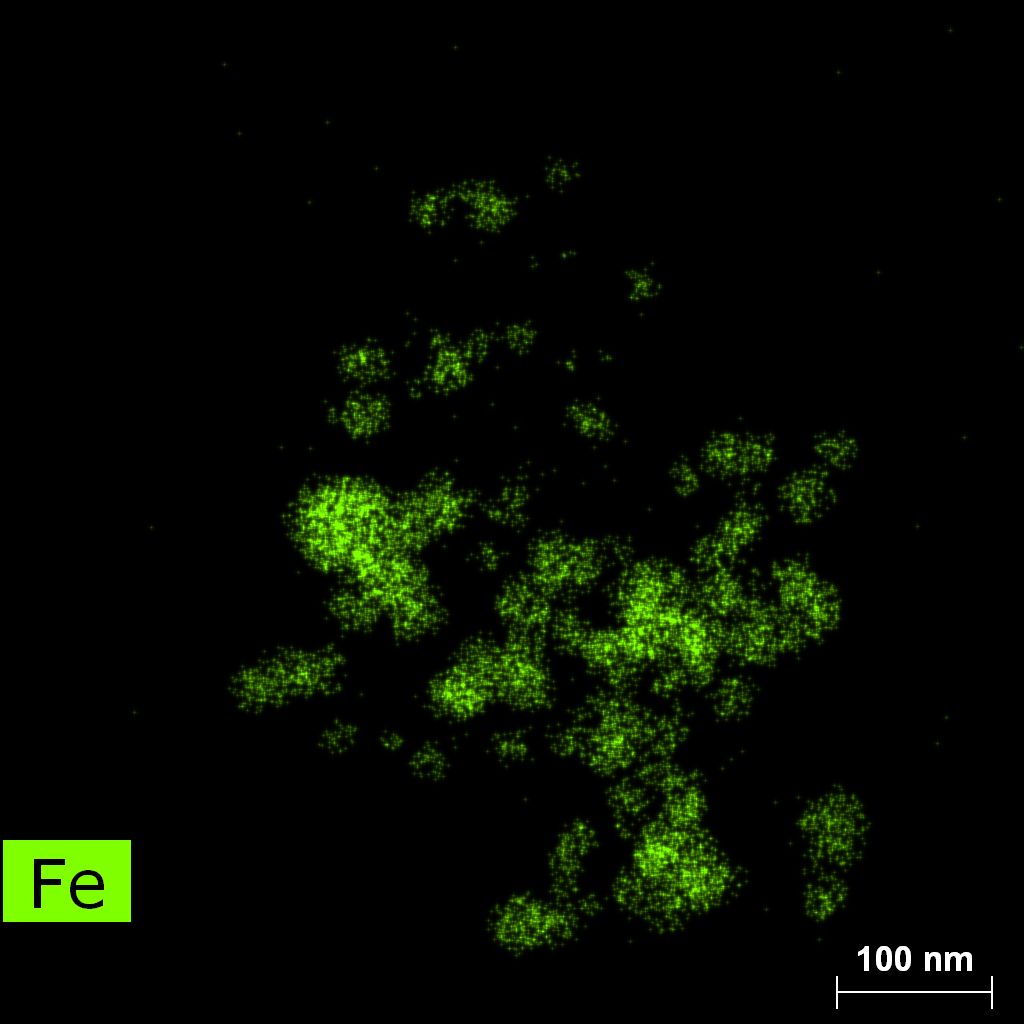
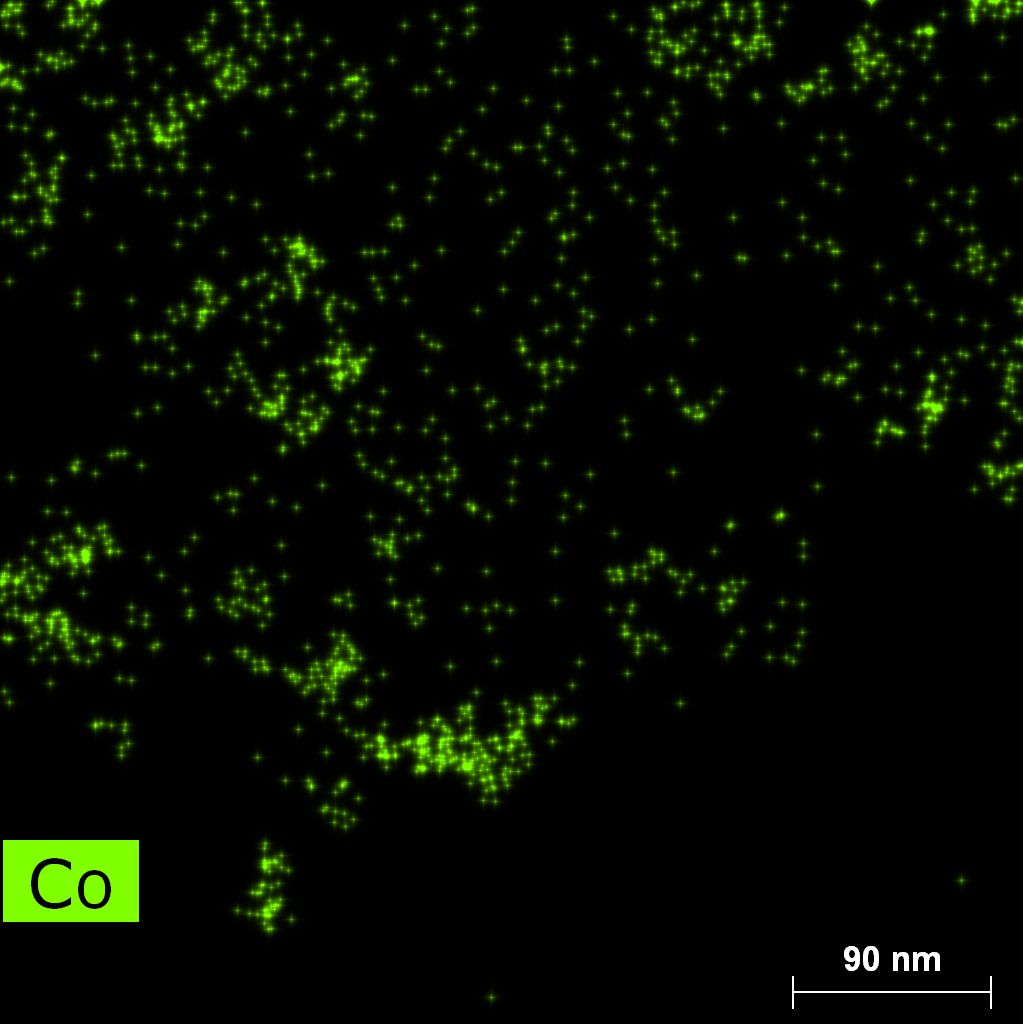
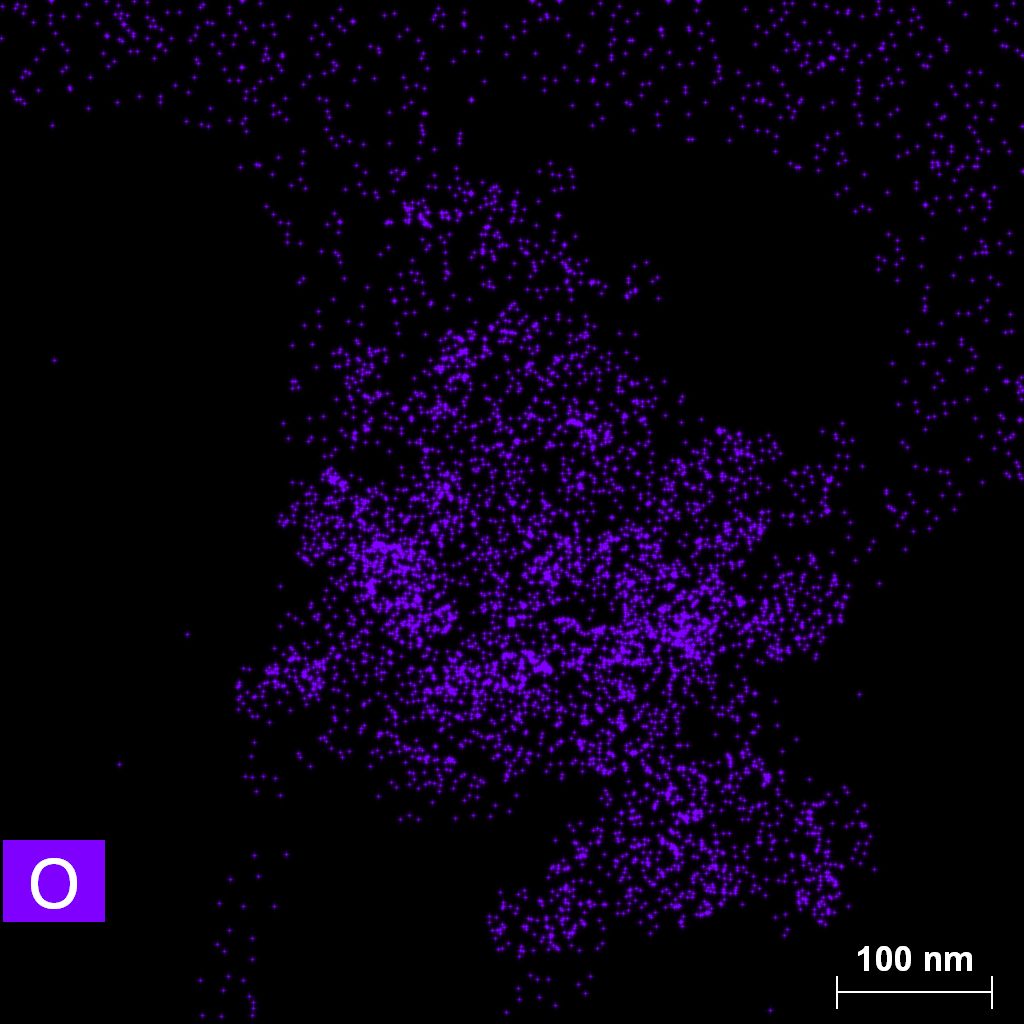
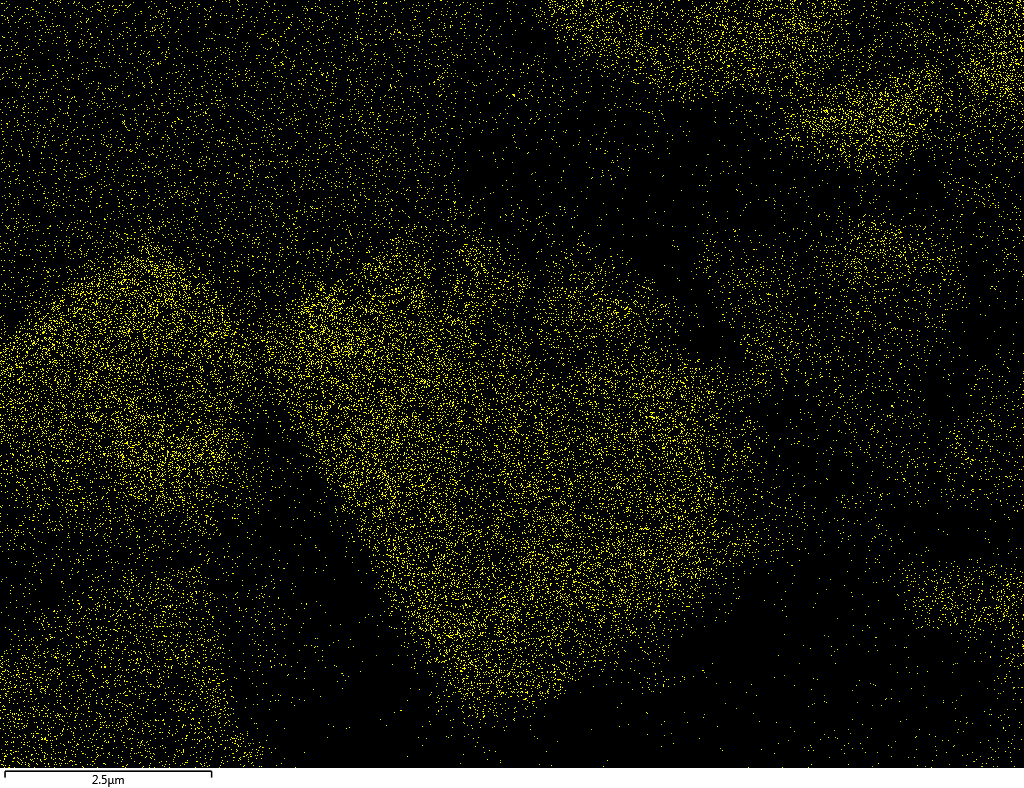
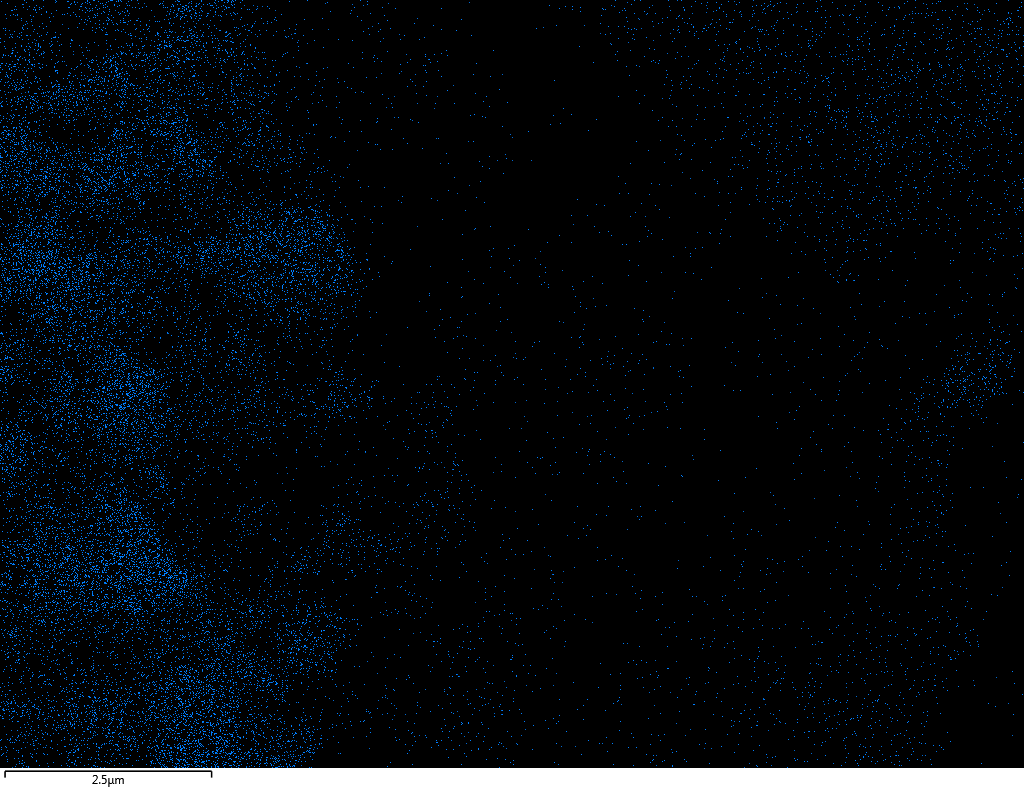
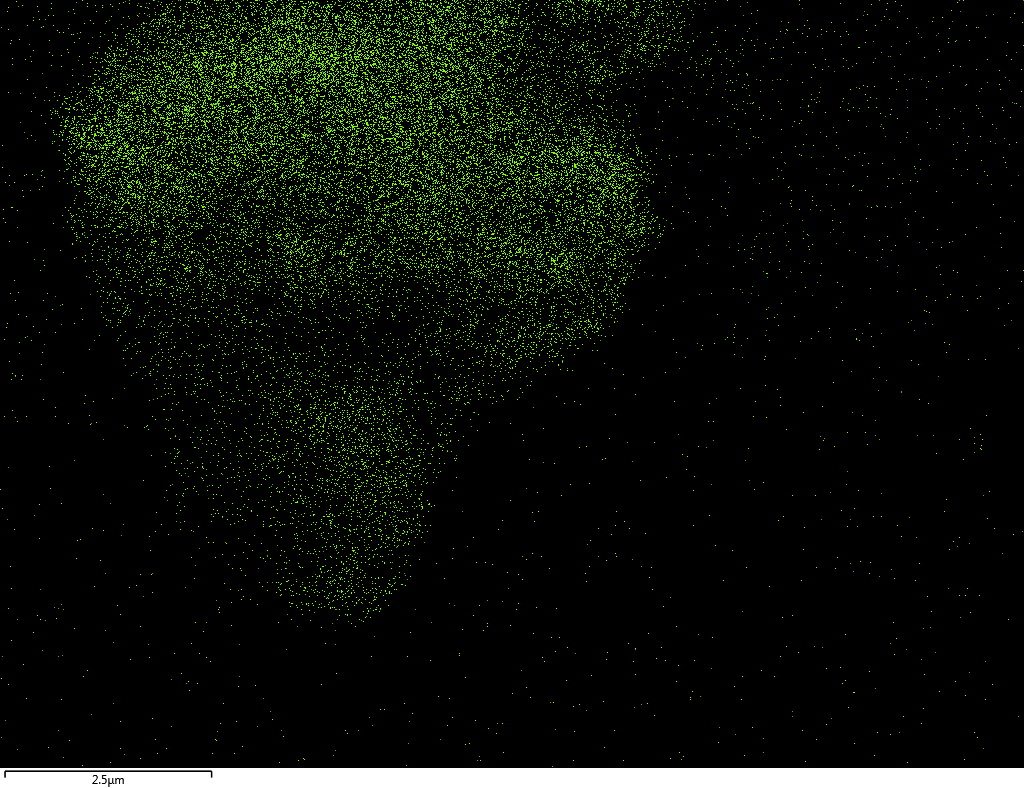
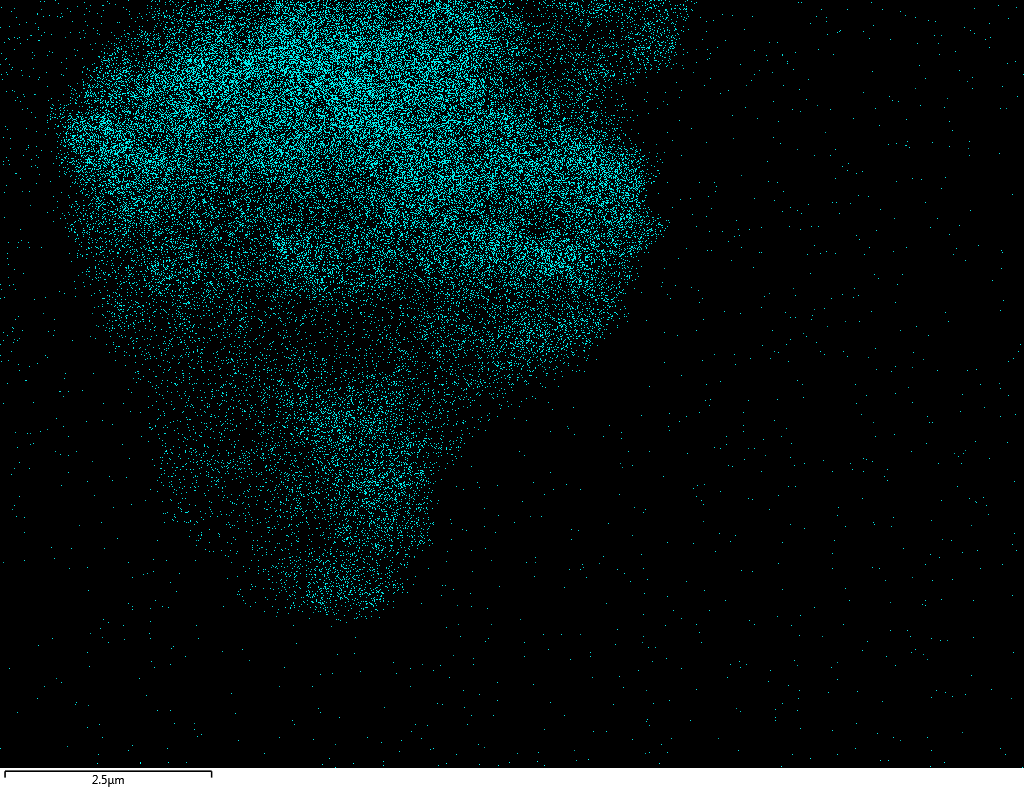
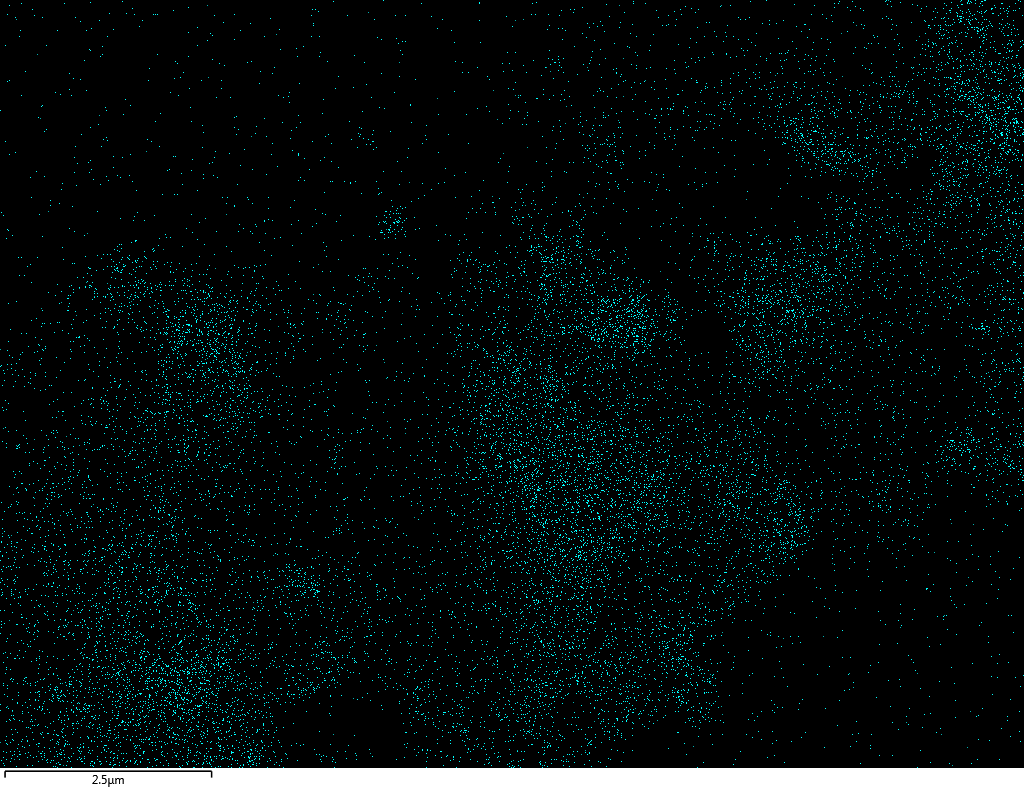
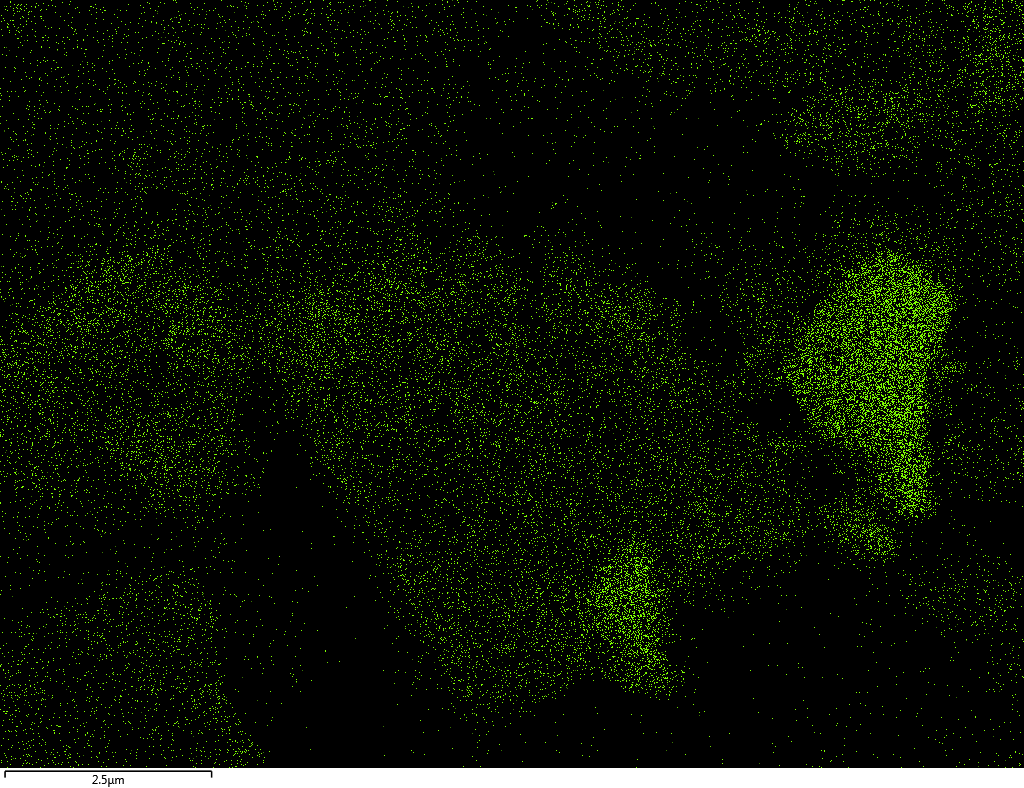
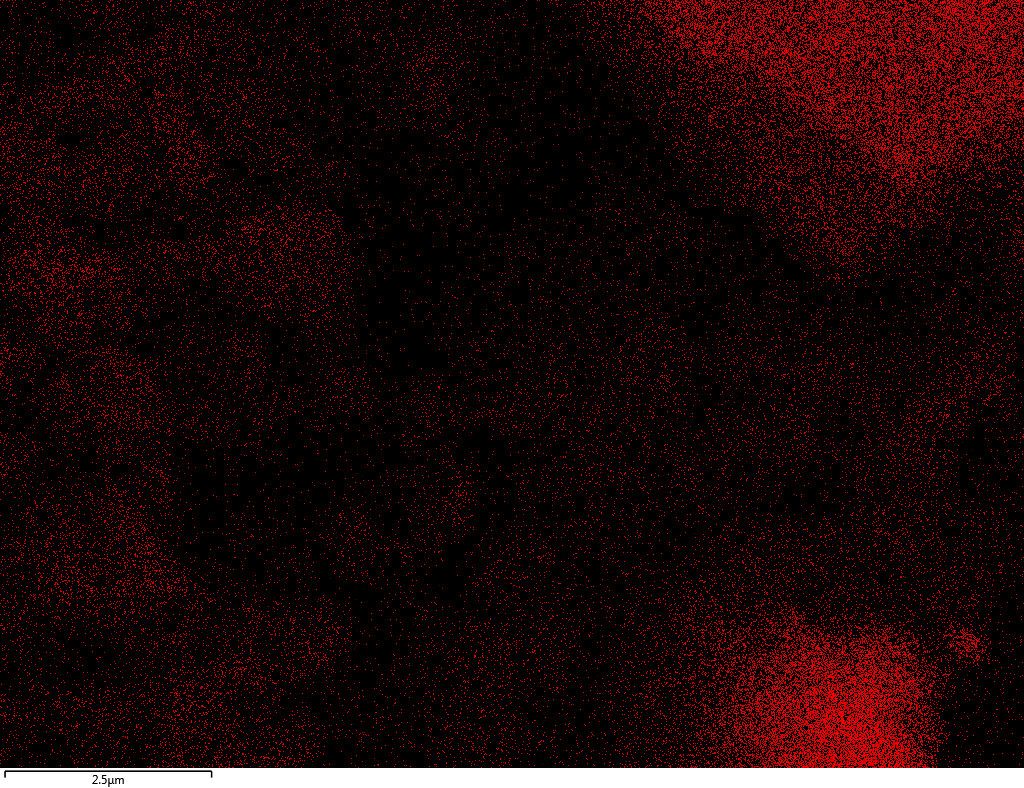
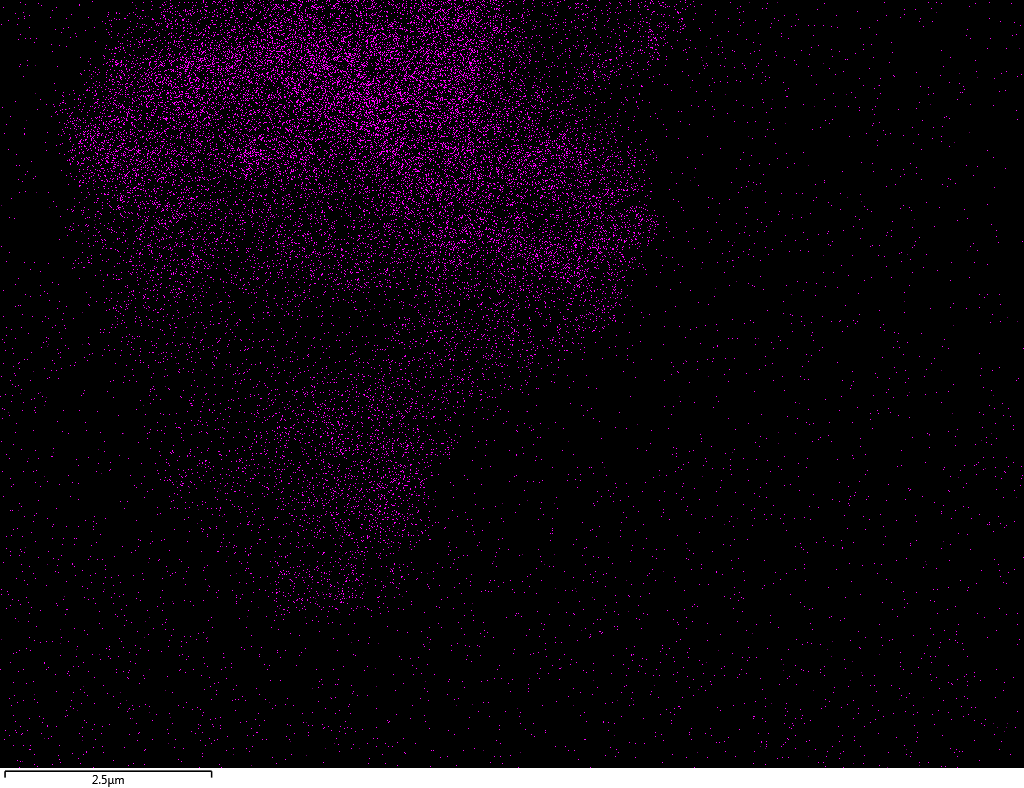
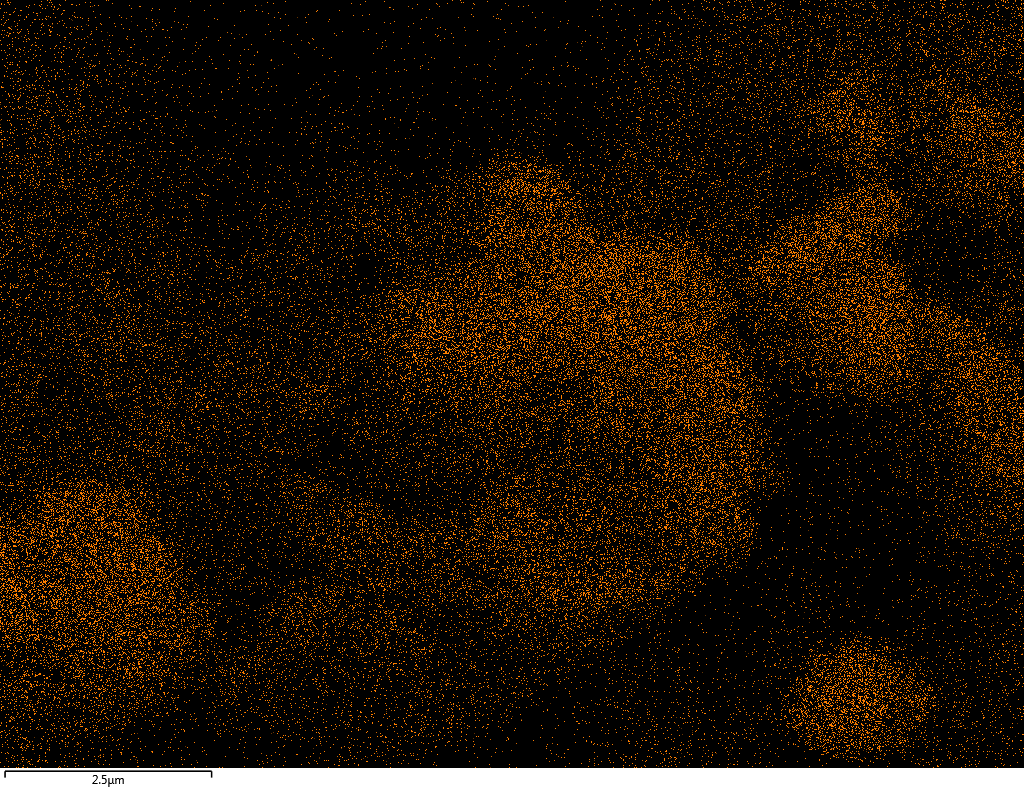
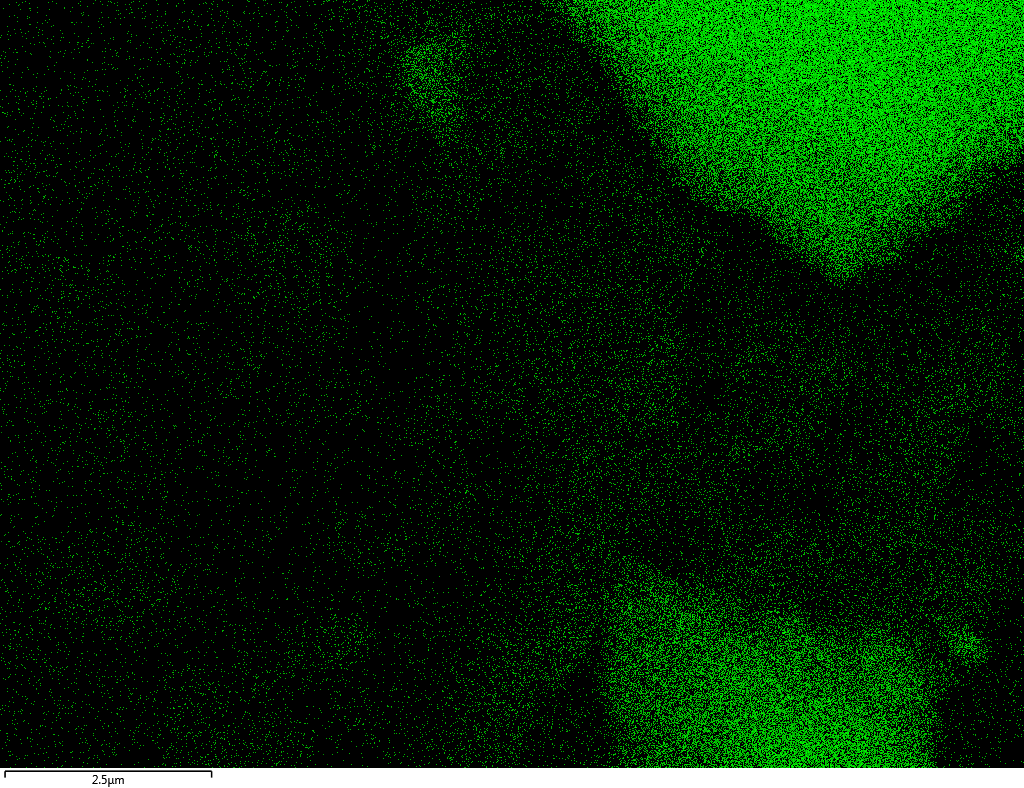
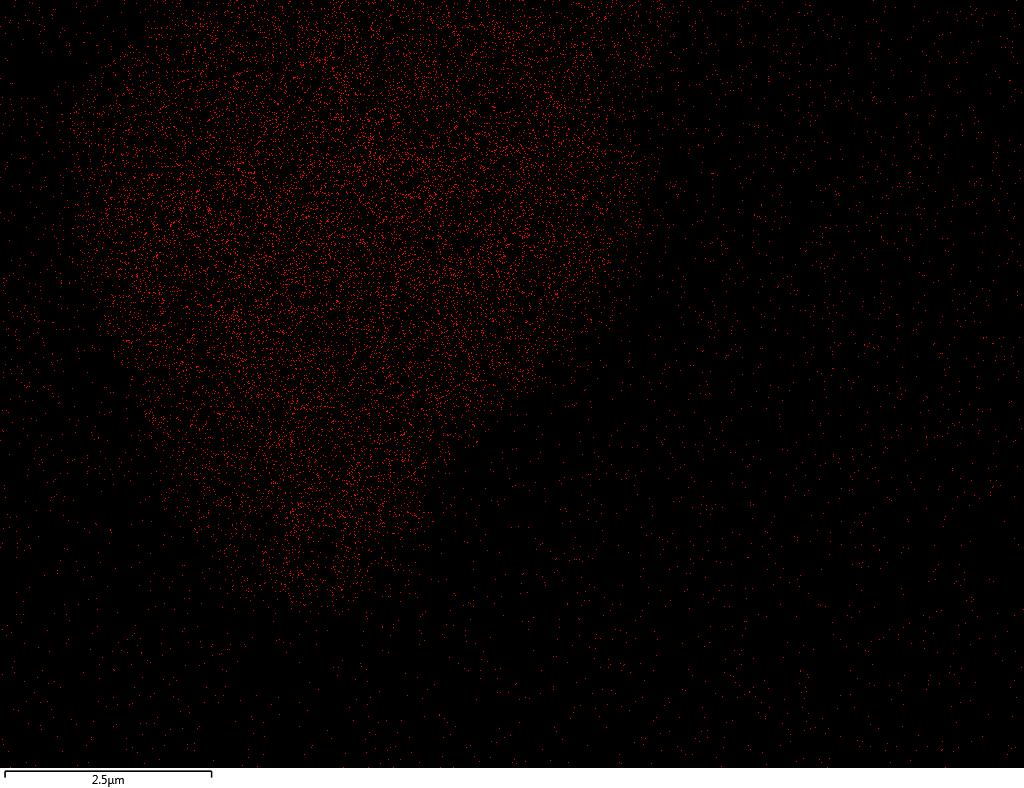
Samples contain B, N, Fe, O elements and up to 0.5 at.% Si impurity. There are homogeniously formed iron nitride particles in sample on BN without ball-milling treatment. In case of ball-milled samples, iron is distributed uniformly among particles.
 |
 |
 |
 |
 |
|
| B | 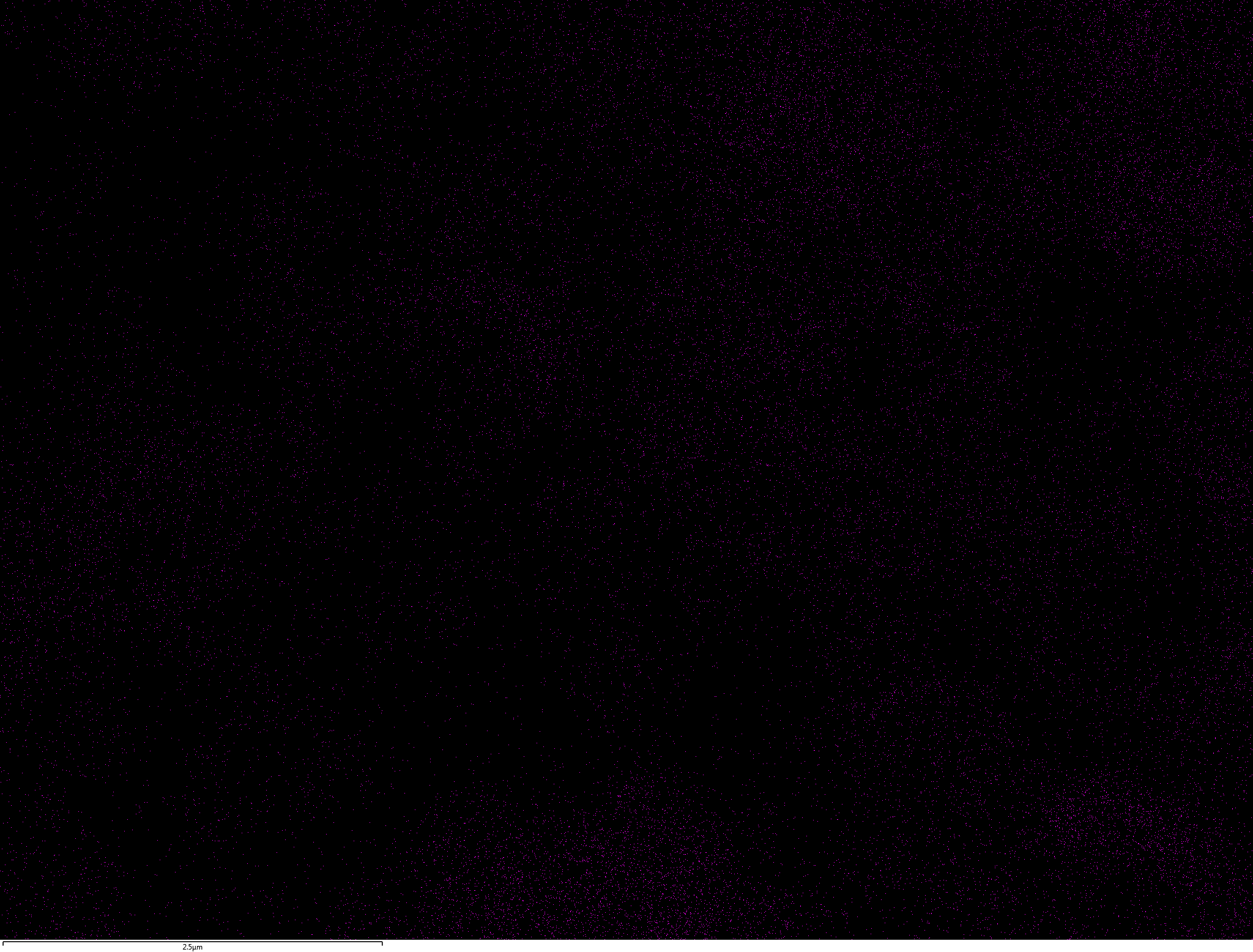 |
 |
 |
 |
 |
| N | 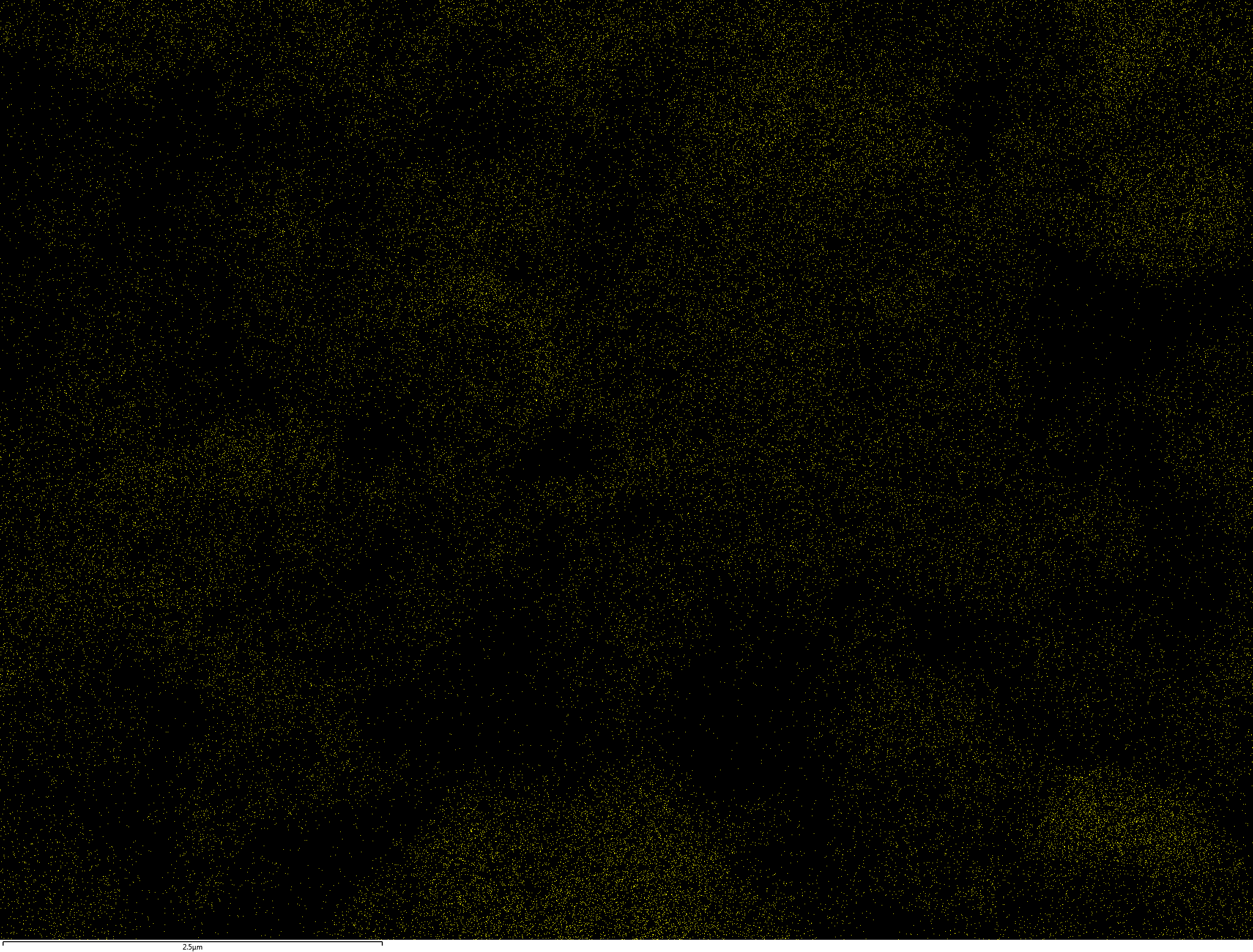 |
 |
 |
 |
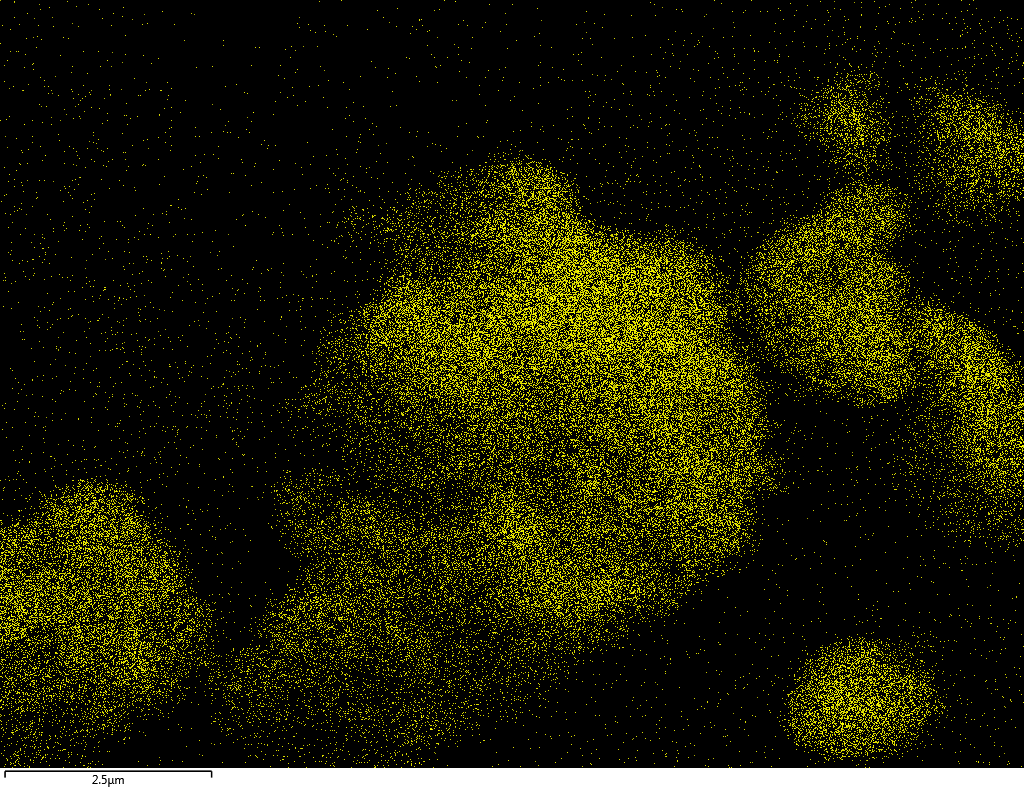 |
| Fe | 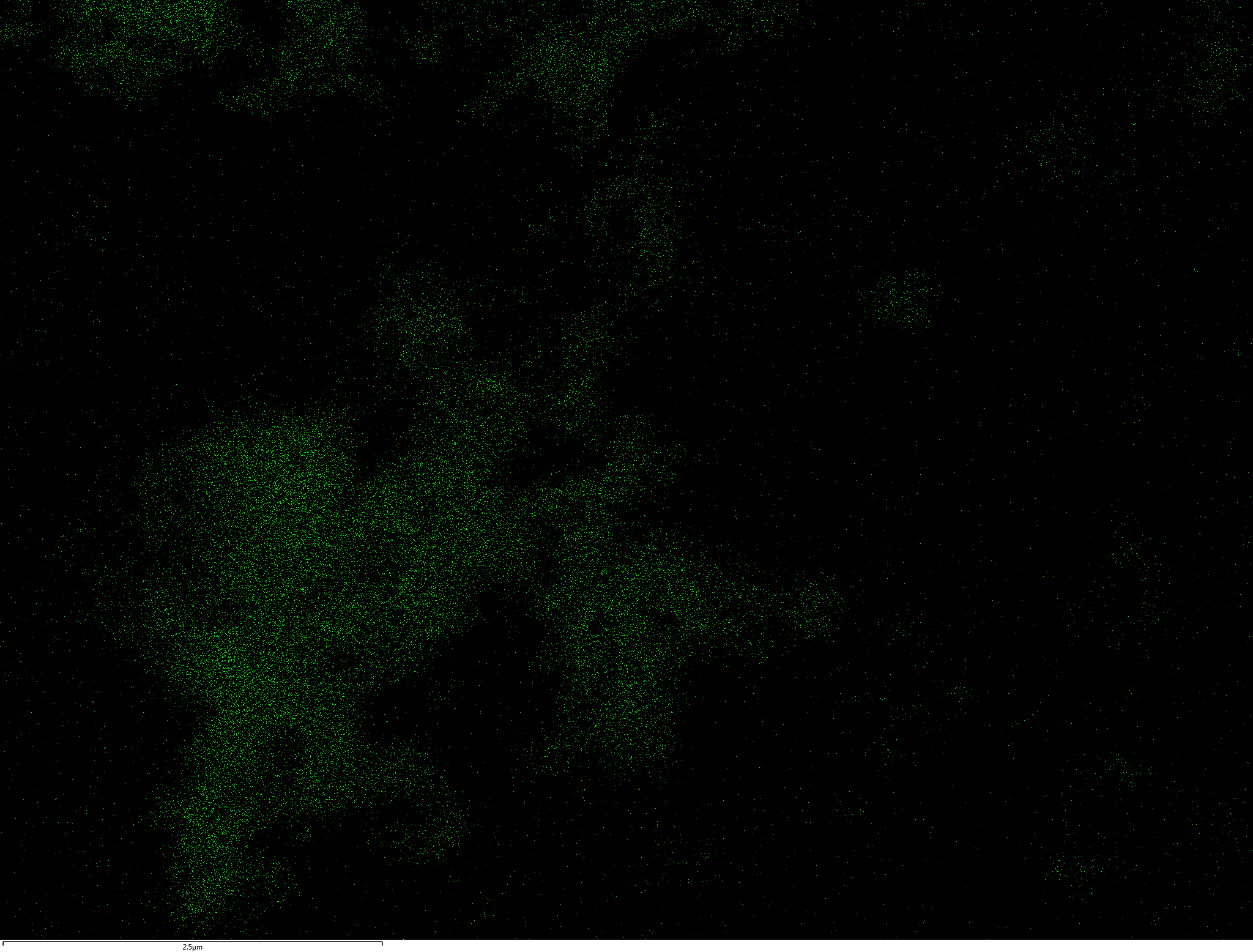 |
 |
 |
 |
 |
| O | 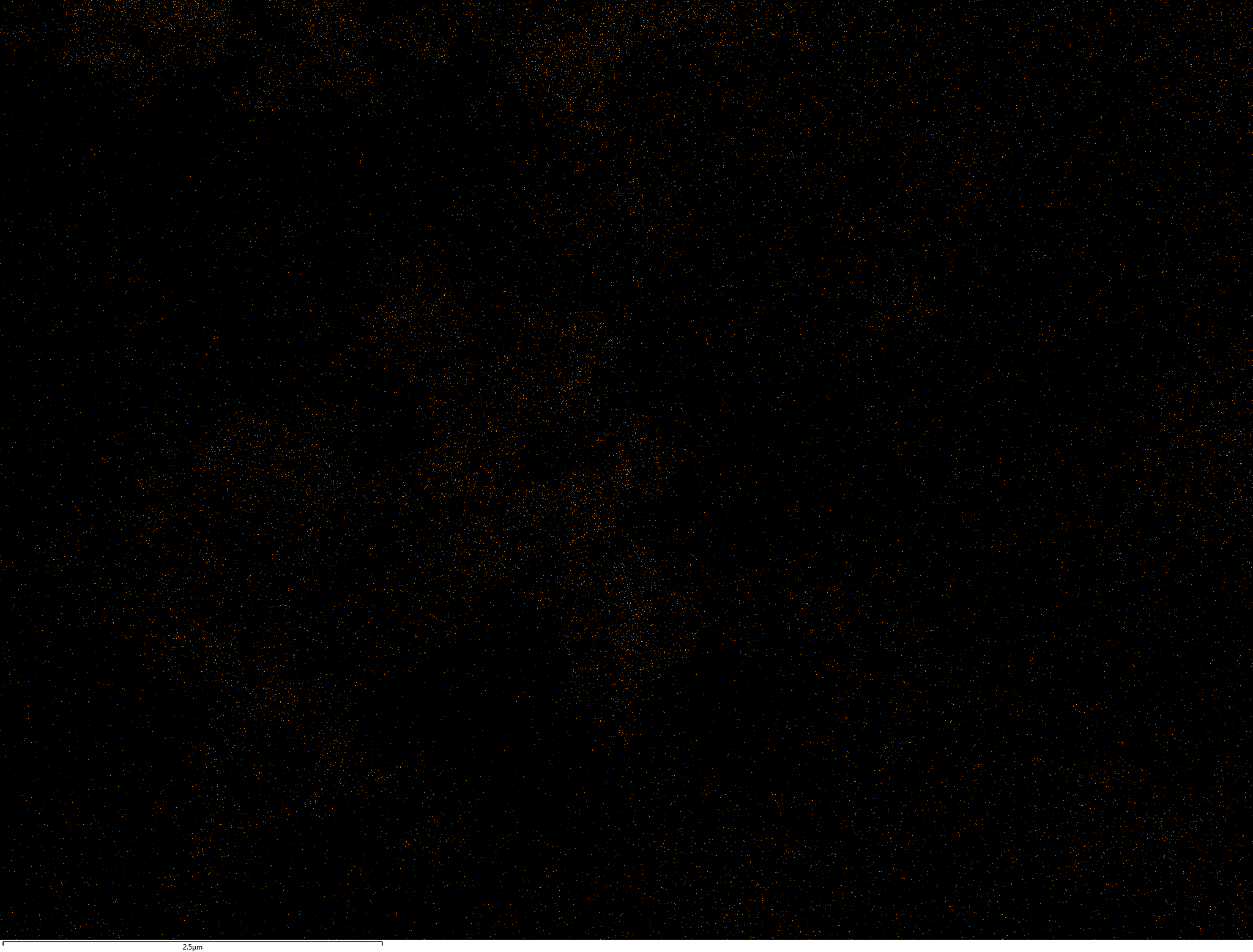 |
 |
 |
 |
 |
| Si | 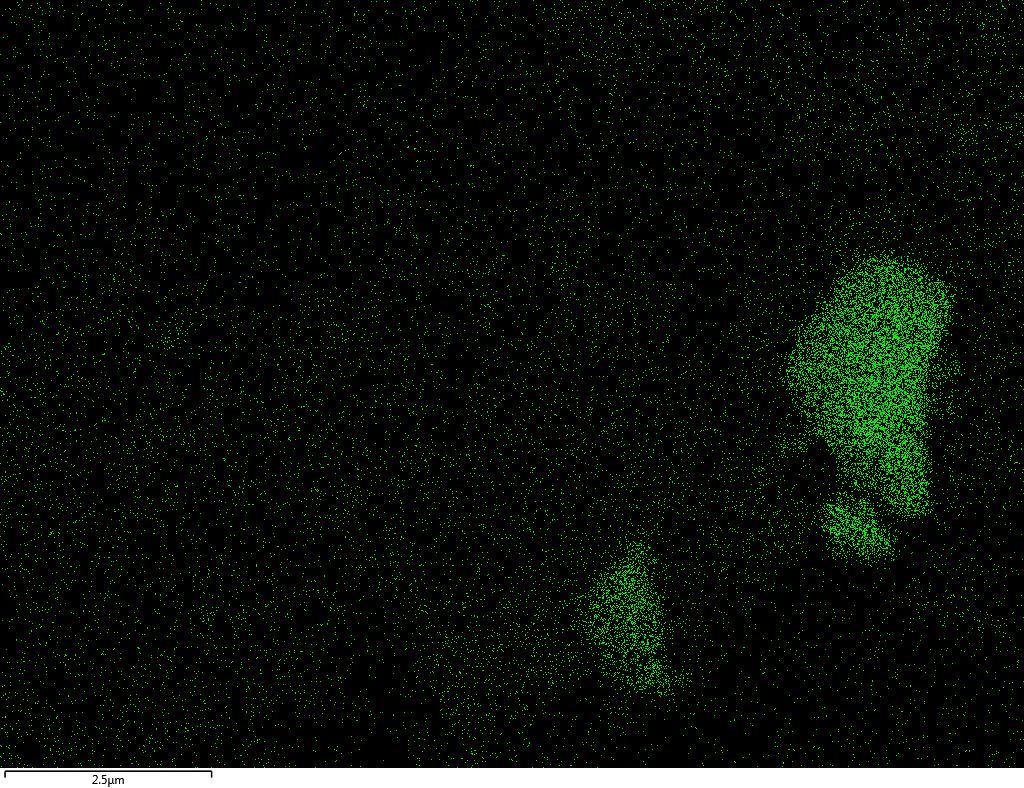 |
 |
 |
||
| dl109: no mill | dl121: b/s=22 | dl122: b/s=33 | dl126: b/s=44 | dl123: b/s=51 |
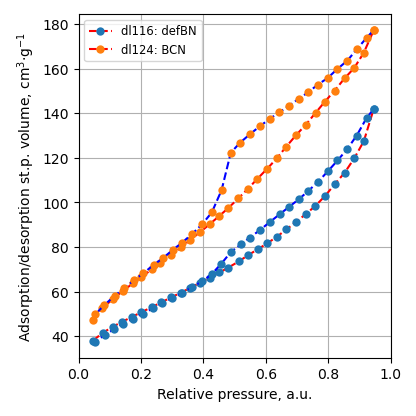
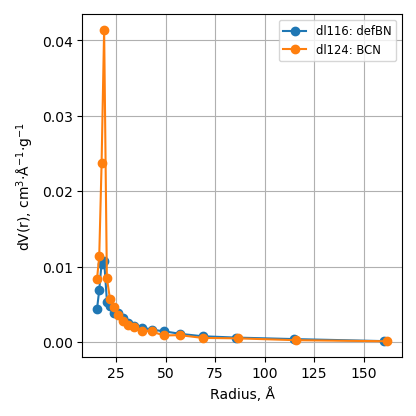
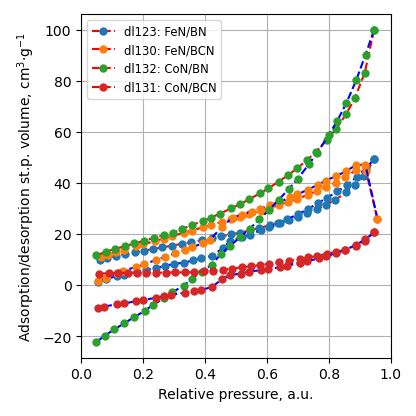
BET
Specific surface area decreases after metal nitride deposition. It is still higher 150 m2/g for ball-milled samples.
| Sample | b/s ratio | Ssp | Ssp,sup |
|---|---|---|---|
| m2/g | m2/g | ||
| dl109 | no mill | 4.787 | 28.726 |
| dl121 | 22 | 196.815 | 309.173 |
| dl122 | 33 | 198.621 | 320.71 |
| dl126 | 44 | 167.740 | 248.579 |
| dl123 | 51 | 230.428 | 224.79 |
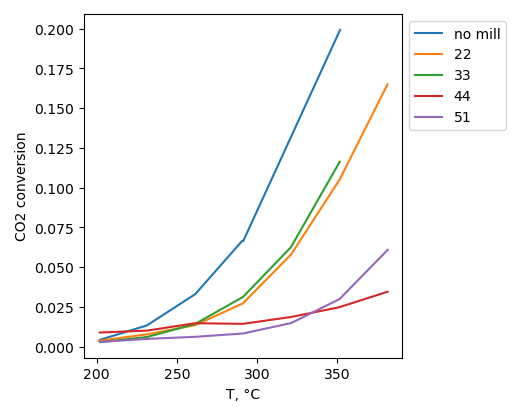
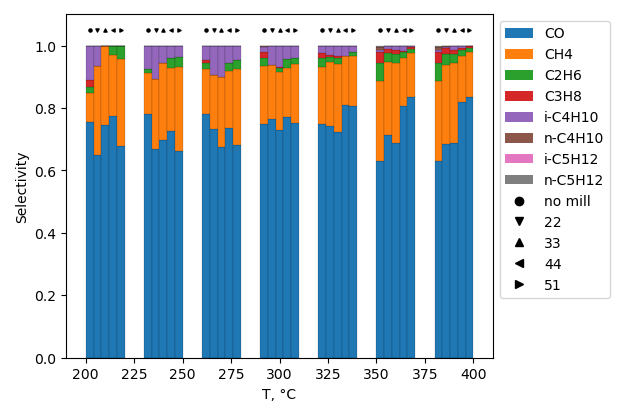
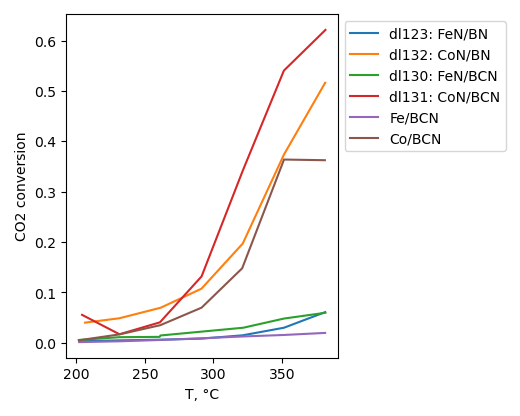
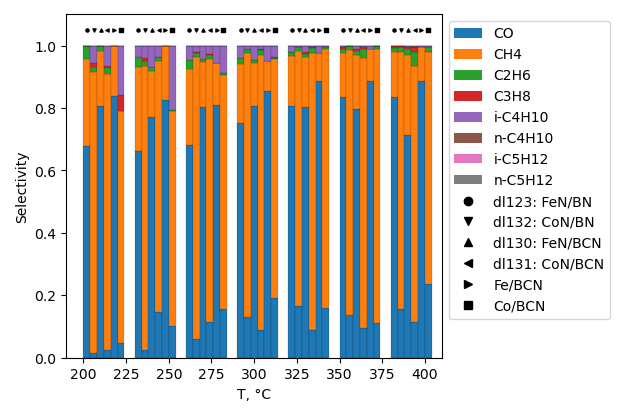
CO2 hydrogenation
CO2 conversion is higher for sample, produced without ball-milling treatment. Selectivity to hydrocarbons is highest for sample with BN ball-milled at 22 b/s ratio at 200°C. However it is highest for non-milled sample at 380°C.